Abstract
Aim
Elevated serum creatinine (sCr) and low estimated glomerular filtration rate (eGFR) are associated with poor outcomes in patients with pulmonary arterial hypertension (PAH) whereas sildenafil treatment improves PAH outcomes. This post hoc analysis assessed the effect of sildenafil on kidney function and links with clinical outcomes including 6-min walk distance, functional class, time to clinical worsening and survival.
Methods
Patients with PAH received placebo or sildenafil 20, 40 or 80 mg three times daily in the SUPER-1 study and open-label sildenafil titrated to 80 mg three times daily (as tolerated) in the extension study.
Results
Baseline characteristics were similar among groups (n = 277). PAH was mostly idiopathic (63%) and functional class II (39%) or III (58%). From baseline to week 12, kidney function improved (increased eGFR, decreased sCr) with sildenafil and worsened with placebo. In univariate logistic regression, improved kidney function was associated with significantly improved exercise and functional class (odds ratios 1.17 [95% CI 1.01, 1.36] and 1.21 [95% CI 1.03, 1.41], respectively, for sCr and 0.97 [95% CI 0.94, 0.99] and 0.97 [95% CI 0.94, 0.99] for eGFR, all P < 0.05). In patients who maintained or improved kidney function, time to worsening was significantly delayed (P < 0.02 for both kidney parameters). Observed trends towards improved survival were not significant. Patients with eGFR <60 (vs. ≥60) ml min–1 1.73 m–2 appeared to have worse survival.
Conclusions
Sildenafil treatment was associated with improved kidney function in patients with PAH, which was in turn associated with improved exercise capacity and functional class, a reduced risk of clinical worsening, and a trend towards reduced mortality.
Keywords: creatinine, glomerular filtration rate, kidney, outcomes, sildenafil
What is Already Known About this Subject
Impaired kidney function, manifested as elevated serum creatinine (sCr) and/or low estimated glomerular filtration rate (eGFR), is associated with poorer outcomes in patients with pulmonary arterial hypertension (PAH).
Therapies for PAH, including sildenafil, improve clinical outcomes for affected patients but the relationship of this improvement with kidney function is poorly characterized.
What this Study Adds
In a post hoc examination of the randomized, controlled SUPER-1 study, sildenafil treatment was associated with improved kidney function in patients with PAH.
Improved kidney function was associated with improved exercise and functional capacity, reduced risk of clinical worsening, and a trend towards reduced mortality.
Introduction
Pulmonary arterial hypertension (PAH) is an uncommon and fatal disease in which increasing pulmonary vascular resistance ultimately culminates in right ventricular failure and death 1. Mildly elevated serum creatinine (sCr) concentration and/or low estimated glomerular filtration rate (eGFR; <60 ml min–1 1.73 m–2) are present in 12%–27% of patients with PAH and are associated with poor outcomes 2–4.
Sildenafil is a phosphodiesterase type 5 inhibitor that is approved for the treatment of PAH. In the 12 week Sildenafil Use in Pulmonary Arterial Hypertension (SUPER-1) study, sildenafil significantly improved 6-min walk distance (6MWD) compared with placebo in patients with World Health Organization (WHO) group I PAH 5. SUPER-1 patients were eligible to enroll in a long-term extension study (SUPER-2) 6.
Because sildenafil treatment improved outcomes in PAH patients, and mildly elevated sCr and/or low eGFR is associated with poor outcomes in patients with PAH, this post hoc analysis assessed the effect of sildenafil on changes in these measures of kidney function in patients enrolled in SUPER-1. Additionally, we assessed the relationships between changes in kidney function parameters and 6MWD, WHO functional class and time-to-clinical worsening (TTCW) in SUPER-1 and survival in SUPER-2. The hypotheses were that sildenafil would improve kidney function and that improved kidney function would lead to improved clinical outcomes (including 6MWD, functional class, TTCW and survival). The sCr results were previously reported in the form of an abstract 7.
Methods
Study design
In the multicentre, randomized, double-blind SUPER-1 study 5, patients ≥18 years of age, with symptomatic PAH that was mostly WHO functional class II or III, and with 6MWD ≥100 and ≤450 m, received placebo or sildenafil 20, 40 or 80 mg three times daily. sCr, 6MWD, WHO functional class and TTCW were assessed at baseline and week 12 in SUPER-1. Clinical worsening was defined as death, lung transplantation, hospitalization for PAH or initiation of additional therapies for PAH (including prostacyclin or bosentan therapy).
In SUPER-2 6, patients received open-label sildenafil titrated to 80 mg three times daily (as tolerated). Survival was tracked until the last enrolled patient had received 3 years of sildenafil treatment (Figure1).
Figure 1.
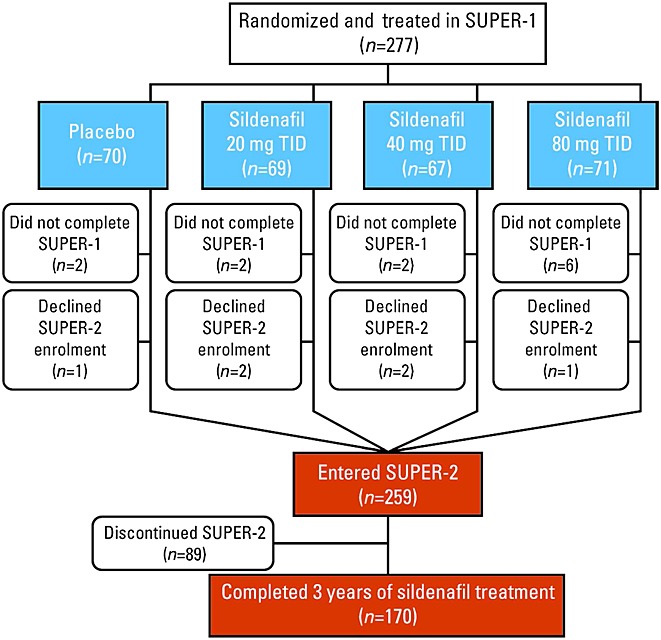
Patient flow. TID 3 times daily
As previously reported, local institutional review boards or independent ethics committees approved the SUPER-1 5 and SUPER-2 6 protocols and written informed consent was obtained from all patients in each trial.
Statistical analysis
Analyses of sCr and eGFR were conducted post hoc. eGFR was calculated using the Modification of Diet in Renal Disease equation 8. Between-treatment differences, 95% CIs and P values for change from baseline to 12 weeks in kidney function measurements were obtained from a parametric analysis of covariance with treatment as a factor and baseline value as the covariate. Changes in kidney function measurements from baseline to 12 weeks were plotted against changes from baseline to 12 weeks in 6MWD and functional class. Pearson correlations were determined.
The relationships between change from baseline to 12 weeks in sCr (per 8.8 µmol l–1 decrease) or eGFR (per 1 ml min–1 1.73 m–2 increase) in patients with ≥10% increase in 6MWD and patients with ≥1 class improvement in WHO functional class were assessed using univariate logistic regression analyses. The relationships between change from baseline to 12 weeks in sCr (per 8.8 µmol l–1 increase) or eGFR (per 1 ml min–1 1.73 m–2 decrease) and time-to-event variables of clinical worsening and all-cause mortality were assessed using univariate Cox regression analyses. Kaplan–Meier curves were generated for TTCW and time to all-cause mortality for patients with improvement or no change vs. deterioration from baseline to last visit in sCr or eGFR. Log-rank tests assessed differences between curves.
Results
Among the 277 patients who were randomized and received ≥1 dose of study medication in SUPER-1, the baseline characteristics of the four treatment groups were not different (Table1). Compared with baseline, sCr increased (worsened) with placebo treatment and decreased with sildenafil treatment (Figure2A). The difference between placebo and sildenafil 80 mg three-times-daily treatment was significant (P = 0.032). Compared with baseline, eGFR decreased (worsened) with placebo treatment and increased with sildenafil treatment (Figure2B). Differences between placebo and sildenafil 40- and 80-mg three-times-daily treatments were significant (P = 0.009 and 0.016, respectively).
Demographic characteristics
| Characteristic | Placebo(n =70) | Sildenafil 20 mg three times daily(n =69) | Sildenafil 40 mg three times daily(n =67) | Sildenafil 80 mg three times daily(n =71) |
|---|---|---|---|---|
| Female, n (%) | 57 (81) | 49 (71) | 47 (70) | 56 (79) |
| Age,*years | 49 ± 17 | 47 ± 14 | 51 ± 15 | 48 ± 15 |
| World Health Organization functional class, n (%) | ||||
| I | 1 (1) | 0 | 0 | 0 |
| II | 32 (46) | 24 (35) | 23 (34) | 28 (39) |
| III | 34 (49) | 40 (58) | 44 (66) | 42 (59) |
| IV | 3 (4) | 5 (7) | 0 | 1 (1) |
| PAH aetiology,†n (%) | ||||
| Idiopathic/heritable PAH | 42 (60) | 44 (64) | 43 (64) | 46 (65) |
| Associated PAH | ||||
| Connective tissue disease | ||||
| Scleroderma | 8 (11) | 9 (13) | 11 (16) | 10 (14) |
| Systemic lupus erythematosus | 4 (6) | 6 (9) | 3 (4) | 6 (8) |
| Other | 10 (14) | 6 (9) | 6 (9) | 5 (7) |
| Repaired systemic-to-pulmonary shunts | 6 (9) | 4 (6) | 4 (6) | 4 (6) |
| 6-min walk distance,* m | 344 ± 79 | 347 ± 90 | 345 ± 77 | 339 ± 79 |
| Serum creatinine,* µmol l–1 (range) | 90.6 ± 20.5 (59–150) | 95.2 ± 26.7‡ (53–200) | 97.1 ± 21.7 (53–165) | 97.5 ± 31.3 (53–248) |
| Estimated glomerular filtration rate,* ml min–1 1.73 m–2 (range) | 69.7 ± 18.5 (33–107) | 69.8 ± 20.8 (23–131) | 66.3 ± 17.8 (34–126) | 66.9 ± 21.2 (18–131) |
PAH, pulmonary arterial hypertension.
Mean ± SD;
Because of rounding, numbers may not add up to 100;
One patient missing an assessment.
Figure 2.
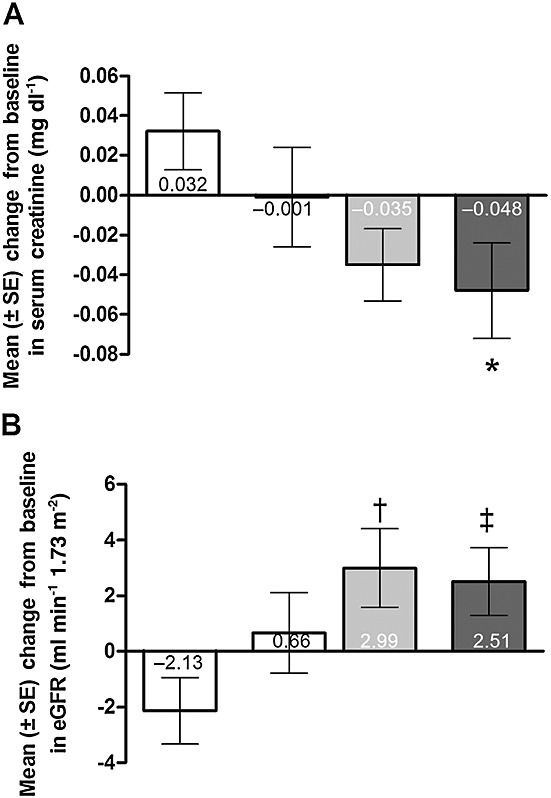
Mean (± SE) change from baseline to SUPER-1 final visit in (A) serum creatinine and (B) estimated glomerular filtration rate (eGFR). *P = 0.032 vs. placebo, least squares mean difference. †P = 0.009 and ‡P = 0.016 vs. placebo, least squares mean difference using analysis of covariance with treatment as the factor and baseline value as the covariate. For the purposes of conversion, 0.02 mg dl–1 is equivalent to 1.76 µmol l–1 sCr.  , placebo (n = 70);
, placebo (n = 70);  , sildenafil 20 mg three times daily (n = 67);
, sildenafil 20 mg three times daily (n = 67);  , sildenafil 40 mg three times daily (n = 65);
, sildenafil 40 mg three times daily (n = 65);  , sildenafil 80 mg three times daily (n = 68)
, sildenafil 80 mg three times daily (n = 68)
Correlations
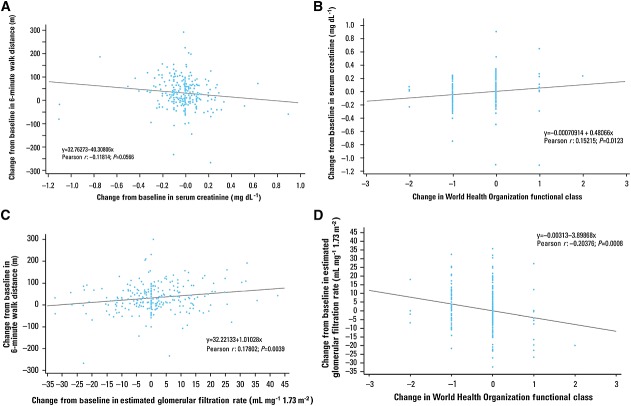
The Pearson correlation coefficient (r) between change from baseline to 12 weeks in sCr and change from baseline in 6MWD was –0.12 (P = 0.057, Figure3A). For change from baseline to 12 weeks in sCr and change from baseline in WHO functional class, r was 0.15 (P = 0.012, Figure3B). Correlations between change from baseline to 12 weeks in eGFR and changes from baseline in 6MWD and WHO functional class were 0.18 (P = 0.004, Figure3C) and –0.20 (P < 0.001, Figure3D), respectively.
Figure 3.
Correlations between change from baseline in serum creatinine and change from baseline in (A) 6-min walk distance and (B) World Health Organization functional class. Correlation between change from baseline in estimated glomerular filtration rate and change from baseline in (C) 6-min walk distance and (D) World Health Organization functional class. For the purposes of conversion, 0.02 mg dl–1 is equivalent to 1.76 µmol l–1 sCr
Weak but significant correlations occurred between change from baseline to 12 weeks in eGFR and improved haemodynamic measurements of pulmonary vascular resistance, mean pulmonary arterial pressure, and cardiac index (Pearson correlation coefficients of –0.21, –0.19 and 0.19, respectively; P ≤ 0.005 for all), but not right atrial pressure (r = –0.09; P = 0.16).
Logistic regression
Improvements in renal function parameters were associated with improved outcomes in univariate logistic regression analysis. Reduction from baseline in sCr (8.8 µmol l–1 decrease) was associated with ≥10% improvement in 6MWD (odds ratio [OR] 1.17, 95% CI 1.01, 1.36, P = 0.04) and ≥1 class improvement in WHO functional class (OR 1.21, 95% CI 1.03, 1.41, P = 0.019). Increase from baseline in eGFR (1 ml min–1 1.73 m–2 increase) was associated with ≥10% improvement in 6MWD (OR 0.97, 95% CI 0.94, 0.99, P = 0.006) and ≥1 class improvement in WHO functional class (OR 0.97, 95% CI 0.94, 0.99, P = 0.007).
Time to clinical worsening and mortality
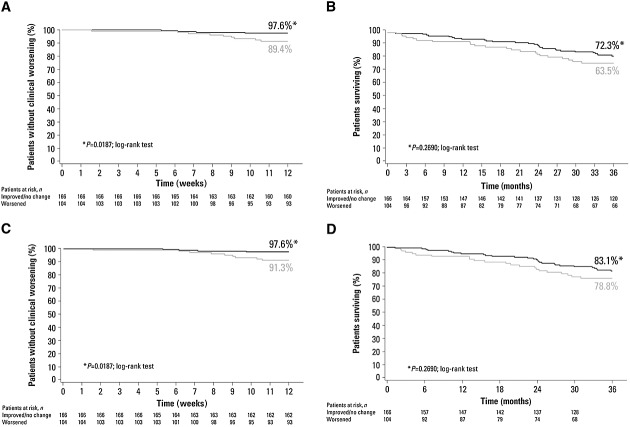
In patients with no change or improved sCr levels, TTCW was significantly delayed (Figure4A) and there was a trend towards reduction in all-cause mortality (Figure4B). Univariate Cox regression analysis of change from baseline in sCr noted that each 0.1 mg dl–1 increase was associated with a higher risk of clinical worsening (hazard ratio [HR] 1.46, 95% CI 1.21, 1.77, P < 0.001) and a trend towards higher risk of mortality (HR 1.10, 95% CI 0.86, 1.41, P = 0.44).
Figure 4.
Kaplan–Meier analysis of (A) time to clinical worsening and (B) time to all-cause mortality by serum creatinine (sCr) status from baseline to week 12; (C) time to clinical worsening and (D) time to all-cause mortality by change in estimated glomerular filtration rate (eGFR) status from baseline to week 12. A–B  , patients with no change or improvement in sCr;
, patients with no change or improvement in sCr;  , patients with worsening sCr. C–D
, patients with worsening sCr. C–D  , patients with no change or improvement in eGFR;
, patients with no change or improvement in eGFR;  , patients with worsening eGFR.
, patients with worsening eGFR.
In patients with no change or increased eGFR, TTCW was significantly delayed (Figure4C) and there was a trend towards reduction in all-cause mortality (Figure4D). Univariate Cox regression analysis of change from baseline in eGFR noted that each 1 ml min–1 1.73 m–2 decrease was associated with a higher risk of clinical worsening (HR 1.09, 95% CI 1.04, 1.14, P = 0.0004) but not all-cause mortality (HR 1.00, 95% CI 0.97, 1.04, P = 0.87).
Patients having baseline eGFR <60 ml min–1 1.73 m–2 appeared to have worse survival than patients with eGFR ≥60 ml min–1 1.73 m–2 (Figure5). Survival appeared higher in patients initially treated with sildenafil compared with those initially treated with placebo for patients with eGFR <60 ml min–1 1.73 m–2. Curves were similar for patients with eGFR ≥60 ml min–1 1.73 m–2.
Figure 5.
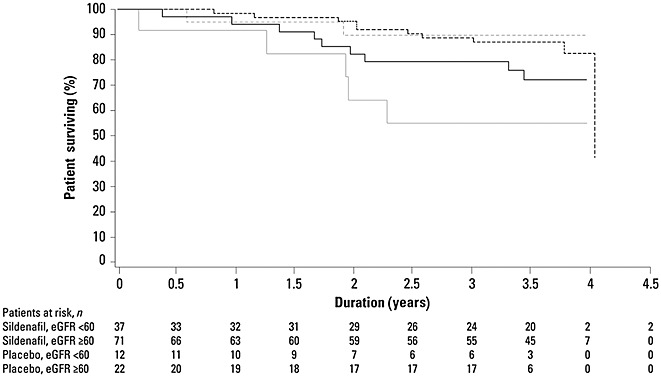
Kaplan–Meier analysis of survival for patients having estimated glomerular filtration rate (eGFR) <60 or ≥60 ml min–1 1.73 m–2 by study treatment (placebo or sildenafil [all doses combined]).  , sildenafil eGFR < 60;
, sildenafil eGFR < 60;  , sildenafil ≥ 60;
, sildenafil ≥ 60;  , placebo < 60;
, placebo < 60;  placebo ≥ 60 ml min–1 1.73 m–2
placebo ≥ 60 ml min–1 1.73 m–2
Discussion
In this post hoc analysis, significant improvements in kidney function, assessed by sCr and eGFR, were observed in sildenafil-treated patients with PAH. Improved kidney function was associated with improved 6MWD and functional class. Although improved kidney function (decreased sCr, increased eGFR) was also significantly associated with a reduced risk of clinical worsening, the observed trends towards improved survival were not significant.
Impaired renal function has been previously shown to be associated with mortality in PAH 3,4. In one study, 19% of PAH patients had renal insufficiency, defined as creatinine clearance <60 ml min–1 1.73 m–2, and reduction in creatinine clearance significantly predicted mortality in both univariate and multivariate analysis 3. sCr weakly but significantly correlated with haemodynamic impairment (including mean pulmonary arterial pressure, pulmonary vascular resistance, cardiac index and cardiac output) whereas creatinine clearance showed weak but significant correlations with right atrial pressure, cardiac output and cardiac index 3. The authors speculated that perhaps a diminished renal perfusion contributes to renal insufficiency in PAH patients. In a second study 4, even mild impairment in kidney function, assessed using sCr or eGFR, predicted increased mortality. Unlike our study, these kidney measures showed a significant interaction with baseline right atrial pressure (but not cardiac index or mean pulmonary arterial pressure). However, neither of these single centre studies assessed a specific treatment for PAH or changes to kidney function parameters with treatment, although baseline values for sCr and eGFR were broadly similar to those of the patients in our cohort. One retrospective analysis of 811 patients who received subcutaneous treprostinil in randomized, placebo-controlled studies determined that each 44 µmol l–1 increase in patients’ baseline creatinine was associated with an approximate two-fold increased risk of death in univariate and multivariate analysis 9. Again, although that retrospective study examined a specific PAH therapy, changes in kidney function with treatment were not assessed as they were in our analysis.
Registry data from a larger patient cohort, the US-based REVEAL registry (n = 2716), support the association of renal insufficiency with death; this association was found in both univariate and multivariate analysis 10. Although the criterion for ‘renal insufficiency’ was investigator judgement rather than specifically defined clinical variables, renal insufficiency at the time of enrolment was included in the proposed prognostic equation. Additionally, the majority of REVEAL patients had prevalent (stable) disease, challenging the ability to extrapolate this conclusion to all PAH patients. No other registries have described the influence of kidney function on outcomes 11–13.
The mechanism by which sildenafil might affect kidney function is not yet established. Beneficial effects of sildenafil in PAH are believed to result from effects of sildenafil on the pulmonary vasculature and right ventricle. Specifically, sildenafil can cause pulmonary artery vasodilation, therefore decreasing afterload on the right ventricle, and can reduce vascular remodelling of the pulmonary artery as well as increase right ventricular inotropy 14. Similar vasodilatory and remodelling effects in the renal vasculature could potentially lead to improved renal function, or might include effects on podocyte function. However, it is plausible that renal effects of sildenafil are independent from effects on PAH or survival.
Endothelin system dysregulation has been postulated to underlie pulmonary and renal vasculopathy. The kidney is a major source of endothelin (ET)-1, and the renal vasculature is highly sensitive to the effects of ET-1 15,16. Sildenafil has been shown to decrease plasma ET-1 concentration in a rat model of PAH 17 and phosphodiesterase type 5 inhibitors like sildenafil can, by promoting the effects of nitric oxide, antagonize the actions of ET-1 18. However, the renal effects of sildenafil in patients with PAH are not fully explored. In this regard, we did not assess proteinuria in this study. Growing evidence would suggest that PDE5 inhibitors may reduce proteinuria 18, which would be of interest to assess in future studies.
Reduction in serum creatinine with sildenafil pretreatment was noted in preclinical models of kidney insult (ischaemia-reperfusion injury 19 and cisplatin-induced nephrotoxicity 20). Sildenafil benefits appeared to derive from inhibition of apoptosis, evidenced by increased expression of inducible and endothelial nitric oxide synthase proteins, as well as effects on other known apoptotic factors. In diabetic nephropathy, sildenafil showed potent antiproliferative effects and reduced extracellular matrix formation 21. Parallels for these actions of sildenafil in the kidney can be found in the lungs of PAH patients. Sildenafil enhances nitric oxide production 22 and aberrant apoptosis, cell proliferation and extracellular matrix formation appear to contribute to PAH pathology 22–24.
It is of note that the response of 6MWD to sildenafil showed a flat dose–response, with little additional effect above 20 mg three times daily, the licensed dose of sildenafil for treatment of PAH. Of interest, the comparisons with placebo and active treatment were only significant above 20 mg three times daily sildenafil, suggesting the dose–response may extend to higher doses for the renal effects.
Study limitations include the post hoc nature of these analyses, the lack of validation of the Modification of Diet in Renal Disease equation in PAH patients, and the lack of direct measures of renal or cardiac function. In addition, the observed trend in survival may be attributable to a small number of deaths on study. N-terminal pro-brain natriuretic peptide values, to allow assessment of an interaction between biomarkers and outcomes, were not obtained in these studies.
In conclusion, sildenafil treatment in patients with PAH improved kidney function assessed by sCr and eGFR and improved kidney function was associated with improved 6MWD, functional class, reduced risk of clinical worsening and a trend towards reduced mortality. Further studies are needed to elucidate the utility of sCr and eGFR as prognostic factors, the mechanism of renal impairment in PAH, and the role of each treatment modality in modifying renal function and prognosis.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare DJW has received research grants from and has served as a consultant to Pfizer, J-LV has served as a consultant to Pfizer and L-JH and JOM are employees of Pfizer.
Data in this manuscript are a post hoc analysis of the SUPER-1 study (clinicaltrials.gov identifier NCT00644605) and its extension study, SUPER-2 (clinicaltrials.gov identifier NCT00159887).
This research was supported by Pfizer Inc. Editorial support was provided by Tiffany Brake, PhD, and Janet E. Matsuura, PhD, from Complete Healthcare Communications Inc. and was funded by Pfizer Inc.
References
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Cedergreen JC, Markin CJ, Wahba IM. Prevalence of chronic kidney disease in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;179:A4864. [Google Scholar]
- Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, Muehling O, Behr J. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest. 2007;131:402–9. doi: 10.1378/chest.06-1758. [DOI] [PubMed] [Google Scholar]
- Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation. 2008;117:2475–83. doi: 10.1161/CIRCULATIONAHA.107.719500. [DOI] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Fleming TR, Galie N, Simonneau G, Ghofrani HA, Oakes M, Layton G, Serdarevic-Pehar M, McLaughlin VV, Barst RJ. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: SUPER-2. Chest. 2011;140:1274–83. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- Webb D, Vachiery J-L, Hwang L-J, Watt S, Maurey J. 2011. Sildenafil reduces serum creatinine in patients with pulmonary arterial hypertension: relationship to clinical outcomes. In: European Respiratory Society Annual Meeting, 2011 September 24–28, ; Amsterdam, Netherlands.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant. 2011;30:982–9. doi: 10.1016/j.healun.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–87. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- Keogh A, Strange G, Kotlyar E, Williams T, Kilpatrick D, Macdonald P, Brown K, Pidoux A, Kermeen F, Steele P, Dalton B, Gabbay E. Survival after the initiation of combination therapy in patients with pulmonary arterial hypertension - an Australian collaborative report. Intern Med J. 2011;41:235–44. doi: 10.1111/j.1445-5994.2010.02403.x. [DOI] [PubMed] [Google Scholar]
- Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–71. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- Vachiery JL, Davenport A. The endothelin system in pulmonary and renal vasculopathy: les liaisons dangereuses. Eur Respir Rev. 2009;18:260–71. doi: 10.1183/09059180.00005709. [DOI] [PubMed] [Google Scholar]
- Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943–55. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med (Maywood) 2006;231:967–73. [PubMed] [Google Scholar]
- Brown K, Dhaun N, Goddard J, Webb D. Potential therapeutic role of phosphodiesterase type 5 inhibition in hypertension and chronic kidney disease. Hypertension. 2014;63:5–11. doi: 10.1161/HYPERTENSIONAHA.113.01774. [DOI] [PubMed] [Google Scholar]
- Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, Na KR, Kim SY, Shin YT, Lee KW. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297:F362–70. doi: 10.1152/ajprenal.90609.2008. [DOI] [PubMed] [Google Scholar]
- Lee KW, Jeong JY, Lim BJ, Chang YK, Lee SJ, Na KR, Shin YT, Choi DE. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology. 2009;257:137–43. doi: 10.1016/j.tox.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kuno Y, Iyoda M, Shibata T, Hirai Y, Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima fatty rats. Br J Pharmacol. 2011;162:1389–400. doi: 10.1111/j.1476-5381.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–36. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther. 2010;126:1–8. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]