Figure 2.
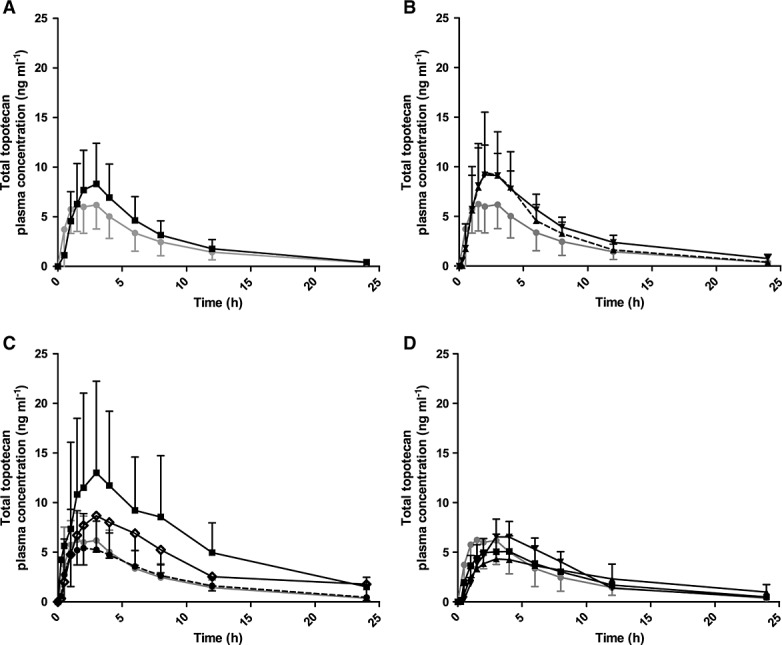
Mean (± SD) plasma concentration (ng ml–1)–time curves of total topotecan following oral administration. Groups A–E represent different groups with normal renal function, with limited previous platinum-based chemotherapy (group A) and with platinum-based chemotherapy (group B, Figure1A), or impaired renal function (group C: mild, group D: moderate and group E: severe, Figure1 B–D). Group A is depicted in all graphs as reference (normal renal function). Oral topotecan doses per group are indicated in mg m−2 day–1. n, number of patients. (A)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group B dose 2.3, n = 12, (B)
Group B dose 2.3, n = 12, (B)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group C dose 1.9, n = 6;
Group C dose 1.9, n = 6;  Group C dose 2.3, n = 13; (C)
Group C dose 2.3, n = 13; (C)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group D dose 1.2, n = 5;
Group D dose 1.2, n = 5;  Group D dose 1.5, n = 6;
Group D dose 1.5, n = 6;  Group D dose 1.8, n = 3; (D)
Group D dose 1.8, n = 3; (D)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group E dose 0.6, n = 5;
Group E dose 0.6, n = 5;  Group E dose 0.8, n = 1;
Group E dose 0.8, n = 1;  Group E dose 1.2, n = 2.
Group E dose 1.2, n = 2.
