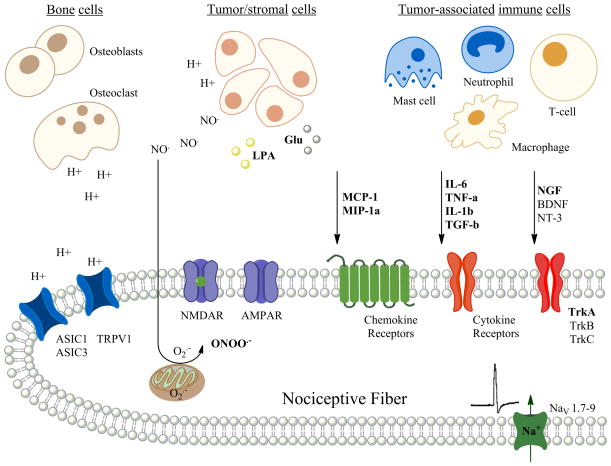
Figure 1.
Diagram of a nociceptive fiber innervating the bone microenvironment demonstrating a number of nociceptive stimuli that act via their receptors that may cause bone cancer pain. Preclinical models have demonstrated that the nociceptive stimuli are released from either the tumor cells themselves or from a number of different tumor-associated immune cells within the bone microenvironment. An addition source of protons and proteases includes the osteoclasts. Preclinical models predict that such nociceptive stimuli may act directly to cause pain via ion channels on the nociceptive fibers or may act via second messengers resulting in the sensitization and increased excitability of the nociceptive fiber within and around the bone. (H+ = proton; NO = nitric oxide; O2− = superoxide; ONOO = peroxynitrate, LPA = lysophosphatidic acid; Glu = glutamate; MCP-1 = monocyte chemottractant protein-1; MIP-1a = macrophage inflammatory protein-1α; IL-6 = interleukin-6; TNF α = tumor necrosis/factor- α; IL-1β = interleukin-1β; TGF-β = transforming growth factor-β; NGF = nerve growth factor; BDNF = brain-derived neurotrophic growth factor; NT-3 = neurotrophin-3; ASIC1 – acid sensing ion channel-1; ASIC3 = acid sensing ion channel-3; TRPV1 = transient receptor potential cation channel-subfamilyV1; NMDAR = N-methyl-D-aspartate receptor; AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; TrkA, B, & C = tyrosine kinase receptors A, B & C)(figure made by AM Symons-Liguori).