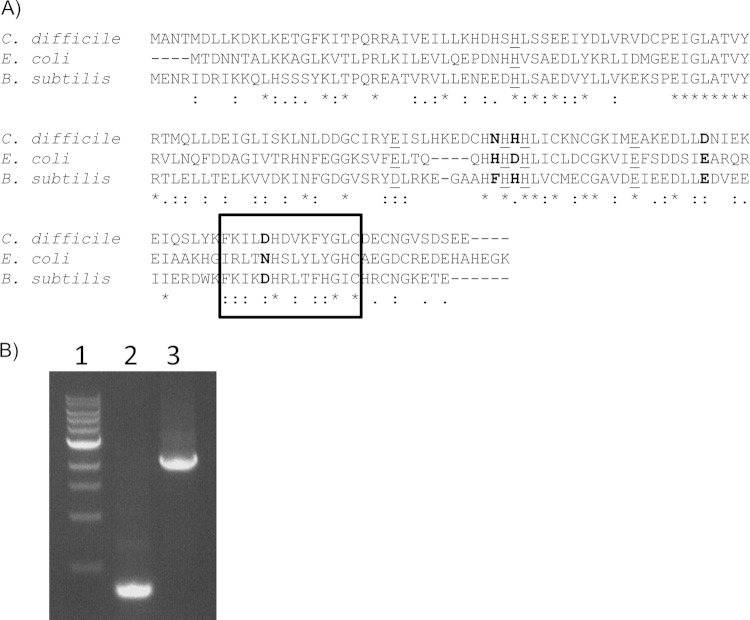
FIG 1.
C. difficile ferric uptake regulator Fur. (A) Alignment of Fur proteins from C. difficile, B. subtilis, and E. coli. The alignment was made with Clustal Omega (18) using default parameters and the following sequence accession numbers: E. coli, NP_415209.1; B. subtilis, NP_390233.2; and C. difficile, YP_001087781.1. The amino acids required for Zn2+ binding are underlined. The amino acids required for Fe2+ binding are in bold. The boxed region is the dimerization domain. (B) PCR Confirmation of wild type and fur::ltrB::erm mutant using primers TE2721 and TE2280, homologous to the 5′ and 3′ ends of the fur gene, respectively. Lane 1, 1-kb ladder; lane 2, wild-type chromosomal DNA; lane 3, fur mutant chromosomal DNA.