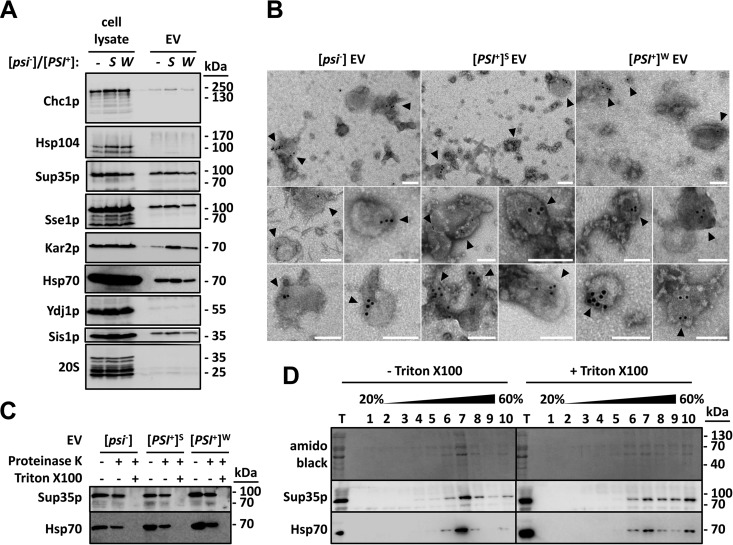
FIG 1 .
Sup35p is localized inside EV produced by [psi−] and [PSI+] cells. (A) The indicated strains were grown to early stationary phase in YPDA medium. Cell lysates and EV were then prepared and analyzed by SDS-PAGE and Western blotting using antibodies against the indicated proteins. (B) EV were fixed with 2% paraformaldehyde, adsorbed onto electron microscopy grids, and permeabilized with 0.02% Triton X-100 for 10 min. Immunogold labeling of Sup35p was then performed using primary polyclonal anti-Sup35p antibody and secondary anti-rabbit 10-nm-gold-conjugated antibodies. Electron microscopy grids were fixed with 1% glutaraldehyde for 5 min at room temperature and visualized by negative-stain electron microscopy. Bars, 100 nm. Arrowheads point to gold-labeled Sup35p-positive vesicles. (C) Purified EV were incubated with or without 2% Triton X-100 for 30 min on ice, before the addition of 0.01 mg ml−1 proteinase K. Reaction mixtures were further incubated for 15 min on ice and stopped with the addition of 2 mM phenylmethylsulfonyl fluoride. An untreated control reaction mixture (without Triton X-100 and without proteinase K) was run in parallel under identical conditions. Reaction products were then analyzed by SDS-PAGE and Western blotting using the indicated antibodies. (D) Purified EV produced by [PSI+]S cells were incubated with or without 1% Triton X-100 for 30 min at 4°C. The mixture was adjusted to 60% sucrose in 20 mM HEPES-OH (pH 7.5) and deposited in the bottom of an ultracentrifuge tube. Equal volumes of 40% and 20% (wt/wt) sucrose solutions in 20 mM HEPES-OH (pH 7.5) were successively layered on top of the EV suspensions. Following centrifugation for 17 h at 150,000 × g and at 4°C, 10 fractions were collected from the top of the gradients and analyzed by SDS-PAGE and Western blotting using the indicated antibodies (T stands for total and represents the input material).