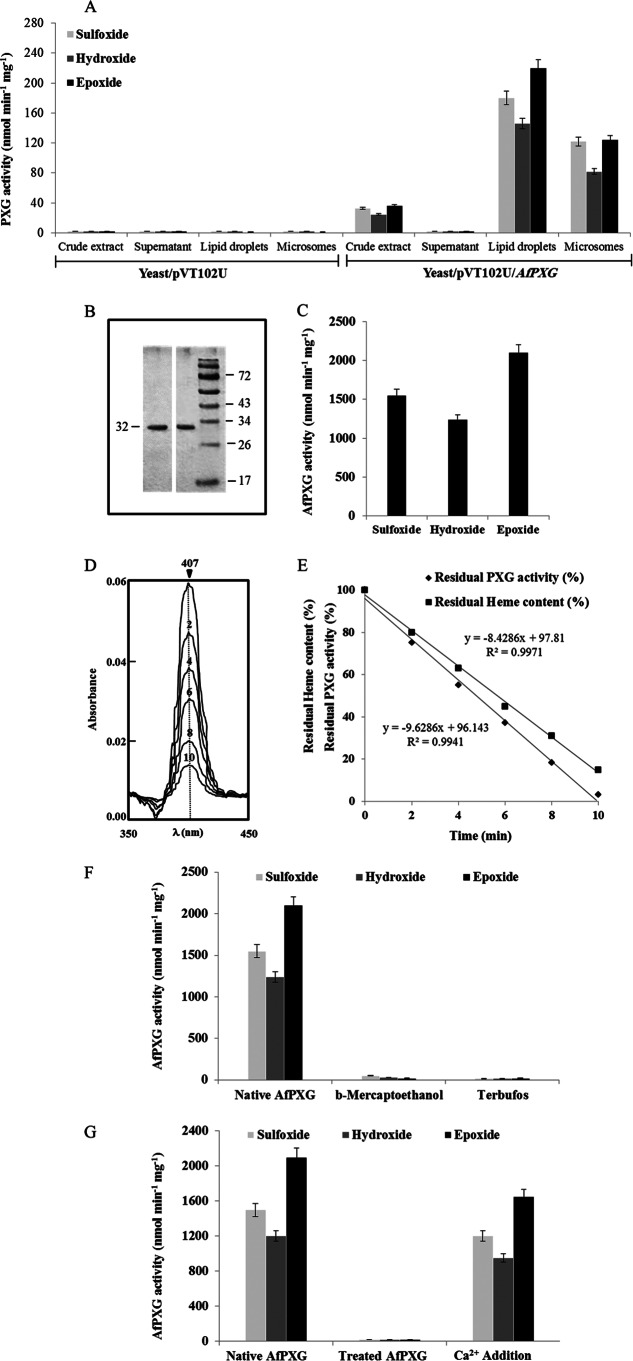
FIG 2.
Biochemical characterization of recombinant AfPXG. (A) PXG activities as the sulfoxidation of thiobenzamide (light gray), the hydroxylation of aniline (dark gray), and the epoxidation of [1-14C]oleic acid (black) were measured in the crude extract, supernatant, lipid droplets, and microsomes of recombinant yeasts containing plasmid pVT102U ligated with the AfPXG insert. Results are means ± SDs (n = 3). (B) SDS-PAGE analysis of purified AfPXG. The purity of the recombinant protein was confirmed by SDS-12% PAGE, followed by Coomassie blue staining (right) and detection of PXG-His by anti-His antibody and an anti-mouse immunoglobulin antibody conjugated to peroxidase (left). Numbers to the left and right of the gels are molecular masses (in kilodaltons). (C) Specific activities of the purified recombinant AfPXG. (D) Sequential scanning of the absolute spectrum of AfPXG obtained on addition of cumene hydroperoxide over the indicated times (in minutes). (E) Correlation between the hydroxylation activity of the purified AfPXG and the disappearance of the heme content. (F) Inhibition of native AfPXG by treatment with β-mercaptoethanol (1 mM) or terbufos (3 mM). (G) Co-oxidation activities for native purified AfPXG and for the Ca2+-deionized AfPXG after extensive dialysis and for AfPXG after dialysis followed by the addition of 1 mM CaCl2 to the medium. All data are the means ± SDs (n = 3).