Fig. 1.
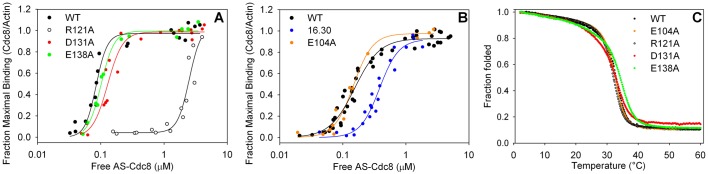
Actin affinity and thermal stability of wildtype and mutant fission yeast tropomyosins. (A,B) Actin affinity of AS-Cdc8p, wildtype and mutants, measured by cosedimentation as described in Materials and Methods (20 mM MOPS pH 7.0, 150 mM NaCl, 2 mM MgCl2, 5 µM actin). (A) AS-Cdc8pwt, Kapp=11.8×106 M−1 (n=3); AS-Cdc8pR121A, Kapp=0.40×106 M−1 (n=2); AS-Cdc8pD131A, Kapp=7.8×106 M−1 (n=3); AS-Cdc8pE138A, Kapp=9.9×106 M−1 (n=2). (B) AS-Cdc8pwt, Kapp=6.6×106 M−1 (n=2); AS-Cdc8pD16A.L30A, Kapp=2.6×106 M−1 (n=3); AS-Cdc8pE104A, Kapp=6.8×106 M−1 (n=2). The binding experiments in A and B were done at different times with different actin preparations. The data for AS-Cdc8pD16A.L30A are reproduced from (Cranz-Mileva et al., 2013) in which the wildtype Kapp=6.3×106 M−1 (n=3). (C) Thermal stability determined by measuring the ellipticity at 222 nm between 0–60°C. The ellipticity at 2°C is normalized to 1. The melting temperature (TM) is defined as the temperature where the normalized ellipticity is 0.5. The observed TM (n=1) are: AS-Cdc8pwt=33°C, AS-Cdc8pD16A.L30A=34°C (Cranz-Mileva et al., 2013); AS-Cdc8pE104A=33°C, AS-Cdc8pR121A=32°C, AS-Cdc8pD131A=33.0°, AS-Cdc8pE138A=34°.
