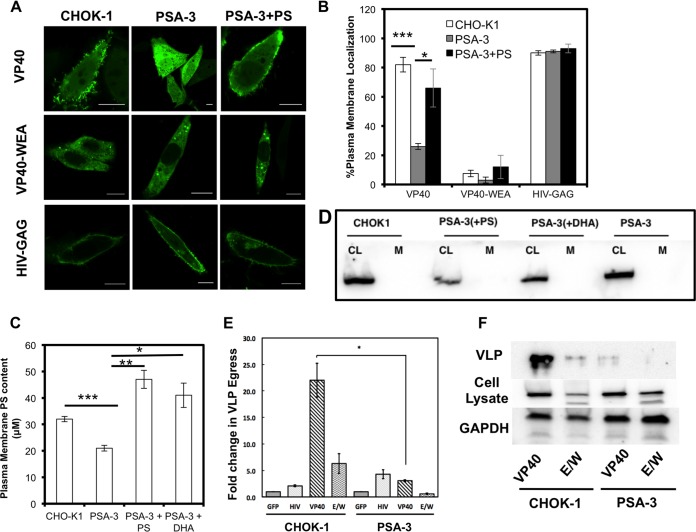
FIG 4.
Plasma membrane PS content regulates VP40 localization, assembly, and viral egress. (A) VP40 lacked significant plasma membrane localization and evidence of egress from the PS-deficient PSA-3 cell line compared to the parent CHOK-1 cell line. VP40 plasma membrane localization and evidence of egress could be restored with the supplementation of POPS in the media. VP40 WEA and HIV-GAG images of CHOK-1 cells, PSA cells, and PSA-plus-PS cells are also shown. Bars, 10 μm. (B) Cells were counted for detectable plasma membrane localization. Experiments were repeated in triplicate to calculate the standard errors of the means (SEM) as indicated. n = 3 independent experiments to determine the SD. Plasma membrane localization of WT VP40 was significantly increased when PSA-3 cells were supplemented with PS compared to PSA-3 cells without PS. (C) The PM PS content was assessed using the enzymatic assay described in Materials and Methods. PSA-3 cells display a nearly 30% reduction in PM PS content, whereas supplementing the cellular media with either PS or DHA restores and increases the PM PS content. (D) Cell lysates (CL) and purified plasma membranes (M) from CHOK-1, PSA-3, and PSA-3 cells treated with either PS or DHA were analyzed via Western blotting using an anticalnexin antibody to assess for ER contamination of the purified plasma membrane. CL, cell lysate; M, plasma membrane. (E) An ELISA demonstrates that PSA-3 cells have a 10-fold reduction in VP40 egress. n = 3 independent experiments to determine the standard errors of the means (SEM). (F) Western blot analysis was performed in triplicate to detect VLPs from CHOK-1 and PSA-3 cells using an anti-EGFP antibody. Cell lysate and GAPDH were also monitored for VP40 expression and a loading control, respectively. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.