Abstract
Improvement in type 2 diabetes after Roux-en-Y gastric bypass (RYGB) has been attributed partly to weight loss, but mechanisms beyond weight loss remain unclear. We performed an ancillary study to the Diabetes Surgery Study to assess changes in incretins, insulin sensitivity, and secretion 1 year after randomization to lifestyle modification and intensive medical management (LS/IMM) alone (n = 34) or in conjunction with RYGB (n = 34). The RYGB group lost more weight and had greater improvement in HbA1c. Fasting glucose was lower after RYGB than after LS/IMM, although the glucose area under the curve decreased comparably for both groups. Insulin sensitivity increased in both groups. Insulin secretion was unchanged after LS/IMM but decreased after RYGB, except for a rapid increase during the first 30 min after meal ingestion. Glucagon-like peptide 1 (GLP-1) was substantially increased after RYGB, while gastric inhibitory polypeptide and glucagon decreased. Lower HbA1c was most strongly correlated with the percentage of weight loss for both groups. At baseline, a greater C-peptide index and 90-min postprandial C-peptide level were predictive of lower HbA1c at 1 year after RYGB. β-Cell glucose sensitivity, which improved only after RYGB, and improved disposition index were associated with lower HbA1c in both groups, independent of weight loss. Weight loss and preserved β-cell function both predominantly determine the greatest glycemic benefit after RYGB.
Introduction
Roux-en-Y gastric bypass (RYGB) results in sustained weight loss (1,2) and has been proposed as a treatment for type 2 diabetes mellitus (T2DM) (3,4). The Diabetes Surgery Study (DSS) randomized patients to lifestyle modification and intensive medical management (LS/IMM) with or without RYGB (5). At 1 year, RYGB patients achieved greater weight loss and superior improvement in HbA1c (5).
In addition to caloric restriction and weight loss, RYGB also changes gastrointestinal transit time and nutrient flow, leading to altered nutrient absorption and gut hormone secretion that may further improve insulin sensitivity and β-cell function (2). To elucidate the mechanisms contributing to improved glycemia after RYGB, we performed an ancillary investigation to the DSS to assess changes in gut hormones and glucagon levels, insulin sensitivity, and insulin secretion in response to enteral stimulus. We hypothesized that greater β-cell function at baseline would be predictive of an improvement in glucose tolerance and correlate with specific gut hormone changes.
Research Design and Methods
Study Participants
Details of the study methods have been published (5,6). Key inclusion criteria included BMI 30–39.9 kg/mg2; HbA1c ≥8% (64 mmol/mol) or ≤14% (130 mmol/mol); and C-peptide >1 ng/mL 90 min after a standardized meal challenge. The 68 patients in this ancillary study were from two of the five study sites that recruited a total of 120 participants.
Interventions
Details of the RYGB and LS/IMM intervention, including the protocol for intensification of medical therapy, have been described (5). Blood specimens were collected before and 15, 30, 60, 90, and 120 min after ingestion of Ensure over 15 min in the morning while upright. Insulin was held the prior evening, and other diabetes medications were held the morning of testing.
Assays
Assays used for determination of hormone concentrations were as follows: 1) insulin and C-peptide were measured using the Immulite Analyzer; 2) total glucagon-like peptide 1 (GLP-1), glucagon-like peptide 2 (GLP-2), and gastric inhibitory polypeptide (GIP) were measured by ELISA (Millipore); and 3) glucagon was measured by radioimmunoassay (Millipore).
Calculations and Statistical Analysis
The insulinogenic index and C-peptide index were quantified as the change in insulin or C-peptide relative to the change in glucose from 0 to 30 min. The insulin secretion rate (ISR) was calculated by deconvolution of C-peptide through the Chronological Series Analyzer software (7,8). HOMA-insulin resistance (IR) was calculated as reported by Matthews et al. (9). The Matsuda index (MI) was calculated as 10,000/(glucose0 × insulin0 × mean glucose × mean insulin)0.5 (10,11). The oral disposition index (oDI) was calculated as the product of the MI and the ratio of insulin secretion relative to glucose. Insulin clearance was calculated as the ratio of fasting C-peptide to fasting insulin. The metabolic clearance rate (MCR) of insulin was calculated as the ratio of the total insulin secretion area under the curve (AUC) to the plasma insulin AUC. β-Cell glucose sensitivity (BCGS) was calculated as the slope between ISR and the corresponding blood glucose, from baseline to glucose at 30 min after meal consumption. AUC was calculated using the trapezoidal rule.
Data are presented as mean values ± SEM. Differences at baseline for continuous variables were assessed with t tests and for categorical variables with χ2 or the Fisher exact test. Change from baseline was calculated for each patient using paired t tests. Between-group differences in change between baseline and 12 months were assessed using two-sample t tests. SAS 9.3 software (SAS Institute Inc., Cary, NC) was used for statistical analysis. A two-sided P value <0.05 was considered statistically significant. There was no adjustment for multiple comparisons.
Results
Clinical Characteristics of Study Participants
Sixty-eight patients were randomized to LS/IMM (41% women) or RYGB (62% women). Mean age (50 ± 1 years), duration of T2DM (10.5 ± 0.7 years), BMI, and HbA1c were similar between the groups (Table 1). Weight loss was greater after RYGB, and diabetes medications were reduced.
Table 1.
Clinical characteristics and changes in glucostatic parameters and gut hormones
| IMM |
P* | RYGB |
P* | P† | |||
|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | ||||
| BMI (kg/m2) | 35.7 ± 0.6 | 32.3 ± 0.7§ | <0.0001 | 35.8 ± 0.5 | 25.7 ± 0.5§ | <0.0001 | <0.0001 |
| Weight (kg) | 105.7 ± 2.9 | 96.1 ± 3.0§ | <0.0001 | 103.2 ± 2.3 | 74.2 ± 2.2§ | <0.0001 | <0.0001 |
| Weight loss (%) | — | 9.2 ± 1.3 | — | — | 28.1 ± 1.3 | — | <0.0001 |
| T2DM medications, n (%) | |||||||
| Orals (except DPP-IV) | 33 (97.1)‡ | 30 (88.2)§ | 0.21 | 27 (79.4)‡ | 14 (41.2)§ | 0.002 | 0.83 |
| Incretin therapy | 10 (29.4) | 31 (91.2)§ | <0.0001 | 5 (14.7) | 7 (20.6)§ | 0.57 | 0.002 |
| Insulin | 15 (44.1)‡ | 15 (44.1) | 1 | 25 (73.5)‡ | 9 (26.5) | <0.0001 | 0.0007 |
| All T2DM medications | 34 (100) | 34 (100)§ | 1 | 34 (100) | 27 (79.4)§ | 0.01 | 0.01 |
| HbA1c (%) | 9.6 ± 0.2 | 7.4 ± 0.3§ | <0.0001 | 9.8 ± 0.2 | 6.4 ± 0.2§ | <0.0001 | 0.0004 |
| HbA1c (mmol/mol) | 81 ± 2.2 | 57 ± 3.3§ | 84 ± 2.2 | 46 ± 2.2§ | |||
| GlucoseF (mg/dL) | 220 ± 8 | 151 ± 8§ | <0.0001 | 236 ± 14 | 117 ± 6§ | <0.0001 | 0.005 |
| Glucose120m (mg/dL) | 252 ± 12 | 159 ± 10§ | <0.0001 | 268 ± 10 | 128 ± 9§ | <0.0001 | 0.01 |
| InsulinF (µIU/mL) | 24.3 ± 3.3 | 20.1 ± 3.1§ | 0.34 | 27.3 ± 4.7 | 7.2 ± 1.6§ | <0.0001 | 0.008 |
| C-peptideF (ng/mL) | 3.4 ± 0.3 | 3.4 ± 0.3§ | 0.82 | 2.8 ± 0.3 | 1.7 ± 0.2§ | 0.0003 | 0.01 |
| C-peptide90m (ng/mL) | 5.4 ± 0.5 | 6.3 ± 0.5 | 0.005 | 4.0 ± 0.4 | 3.8 ± 0.3 | 0.60 | 0.02 |
| 30-min insulinogenic index (μIU × dL × mL−1 × mg−1) | 0.30 ± 0.05 | 0.35 ± 0.06§ | 0.40 | 0.24 ± 0.04 | 0.51 ± 0.09§ | 0.006 | 0.04 |
| 30-min C-peptide index (ng × dL × mL−1 × mg−1) | 0.041 ± 0.01 | 0.036 ± 0.006§ | 0.69 | 0.024 ± 0.003 | 0.065 ± 0.01§ | 0.0003 | 0.0025 |
| Insulin clearance, CF/IF (ng/µIU) | 0.20 ± 0.02 | 0.31 ± 0.04 | 0.01 | 0.17 ± 0.02 | 0.40 ± 0.04 | <0.0001 | 0.046 |
| MCR (kg × mL × min−1) | 353 ± 29 | 1,163 ± 96 | <0.0001 | 281 ± 28 | 1,162 ± 79 | <0.0001 | 0.51 |
| HOMA-IR (mmol × µIU × L−2) | 12.5 ± 1.9 | 7.6 ± 1.3§ | 0.04 | 14.7 ± 2.1 | 2.2 ± 0.5§ | <0.0001 | 0.01 |
| MI [1/(mmol/L)2 × (pmol/L)2] | 2.2 ± 0.2 | 5.0 ± 0.9§ | 0.001 | 2.7 ± 0.5 | 9.1 ± 1.0§ | <0.0001 | 0.004 |
| oDI (IAUC/glucoseAUC × MI) | 0.23 ± 0.02 | 0.61 ± 0.08§ | <0.0001 | 0.20 ± 0.02 | 1.35 ± 0.16§ | <0.0001 | <0.0001 |
| BCGS (pmol × kg−1 ×min−1)/(mg/dL) | 18.6 ± 4.2 | 19.7 ± 4.0 | 0.74 | 11.5 ± 2.4 | 31.2 ± 5.0 | 0.002 | 0.007 |
| Adiponectin (mg/mL) × 103 | 3.2 ± 0.5 | 3.6 ± 0.6§ | 0.13 | 2.8 ± 0.5 | 6.6 ± 0.9§ | <0.0001 | <0.0001 |
| GLP-1 (pmol/L) | 23.6 ± 2.3 | 24.0 ± 2.4§ | 0.81 | 19.1 ± 2.2 | 13.9 ± 1.6§ | 0.02 | 0.05 |
| GIP (pg/mL) | 94.8 ± 7.6 | 103.3 ± 14.4 | 0.57 | 115.6 ± 17.0 | 83.1 ± 6.9 | 0.06 | 0.07 |
| GLP-2 (ng/mL) | 7.0 ± 0.6 | 5.7 ± 0.5§ | 0.03 | 5.7 ± 0.5 | 4.1 ± 0.6§ | 0.005 | 0.63 |
| Glucagon (pg/mL) | 93.0 ± 7.5 | 80.8 ± 5.1§ | 0.02 | 83.8 ± 4.9 | 66.7 ± 3.3§ | 0.0005 | 0.47 |
| InsulinF/glucagonF | 7.3 ± 1.1 | 6.2 ± 0.9§ | 0.39 | 8.2 ± 1.6 | 2.8 ± 0.6§ | 0.0001 | 0.02 |
| Insulin120m/glucagon120m | 9.9 ± 1.2 | 11.8 ± 1.6§ | 0.22 | 12.3 ± 2.9 | 4.7 ± 0.9§ | 0.003 | 0.001 |
| InsulinAUC/glucagonAUC | 20.4 ± 2.8 | 23.7 ± 3.2 | 0.27 | 22.5 ± 4.8 | 17.4 ± 2.6 | 0.11 | 0.05 |
Values are mean ± SEM or n (%).
AUC is calculated from 0 to 120 min.
Molar ratios for insulin/glucagon are presented.
C, C-peptide; DPP-IV, dipeptidyl peptidase-4; F, fasting; I, insulin.
*P = within group change.
†P = difference in the change over time between groups.
‡P < 0.05 between groups at baseline.
§P < 0.05 between groups at 12 months.
Glucostatic Parameters
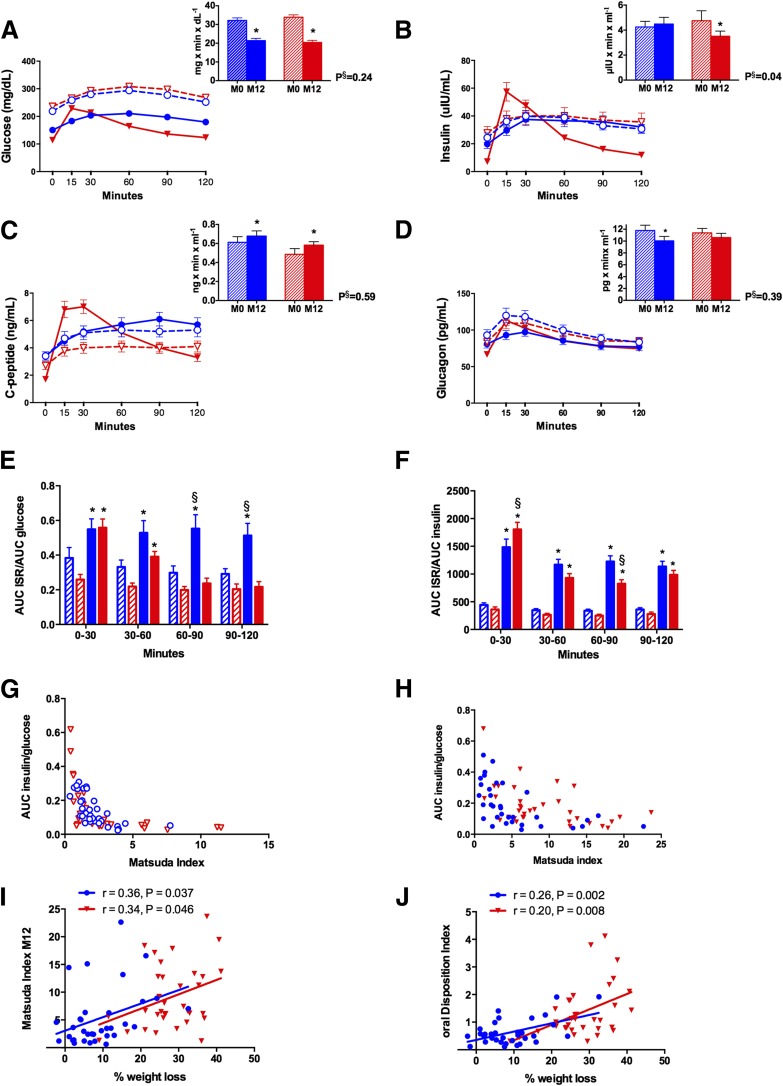
After 1 year, RYGB patients had significantly lower fasting glucose and HbA1c (Table 1). Glucose AUC decreased comparably after both interventions, but postprandial glucose after RYGB peaked earlier and decreased more precipitously (Fig. 1). Fasting insulin and C-peptide decreased only after RYGB. Despite greater early postprandial insulin levels, the insulin AUC was lower after RYGB; the C-peptide AUC increased in both groups (Fig. 1). The insulinogenic index and C-peptide index both increased more than twofold only after RYGB.
Figure 1.
Glucostatic and pancreatic hormone changes. Changes in glucose (A), insulin (B), C-peptide (C), and glucagon (D). E: Changes in insulin secretion relative to glucose. F: Changes in metabolic insulin clearance. G: MI at baseline. H: MI after 1 year. I: Regression analysis of MI after 1 year and percentage of weight loss. J: Regression analysis of oDI after 1 year and percentage of weight loss. LS/IMM is depicted in blue and RYGB is depicted in red. Open shapes and dashed lines denote baseline values, and closed shapes and solid lines denote values at 1 year. Hatched bars denote baseline values, and solid bars denote values at 1 year. AUC is calculated from 0 to 120 min and is expressed ×103. M0, month 0 (baseline); M12, month 12. *P < 0.05 for within-group change. §P < 0.05 for difference in change between groups.
Fasting glucagon decreased in both groups. The glucagon AUC also decreased for both groups but was only significant after LS/IMM because the overall decrease in glucagon was offset by the early postprandial increase after RYGB (Fig. 1). After RYGB, adiponectin increased twofold and correlated with weight loss (r = 0.23; P = 0.003) but was unchanged after LS/IMM.
Insulin clearance increased 50% after LS/IMM and more than twofold after RYGB. There was greater improvement in early MCR (0–30 min) after RYGB (Table 1 and Fig. 1). The ISR increased in LS/IMM patients for all postprandial time points, even after adjusting for concurrent glucose (Fig. 1). Although the ISR from 0 to 30 min was greater after RYGB than after LS/IMM, the rate was no longer different between the groups after adjusting for glucose (Fig. 1). However, there was a precipitous decline in ISR from 60 to 120 min postprandially in RYGB patients that was not observed with LS/IMM (Fig. 1).
There were greater changes in HOMA-IR and MI after RYGB (Fig. 1). A higher MI correlated with a greater percentage weight loss for both LS/IMM and RYGB (Fig. 1). The oDI increased threefold for LS/IMM and sixfold for RYGB and also correlated with the percentage of weight loss (Fig. 1). BCGS increased significantly only for RYGB and was relatively unchanged in LS/IMM.
Gut Hormones
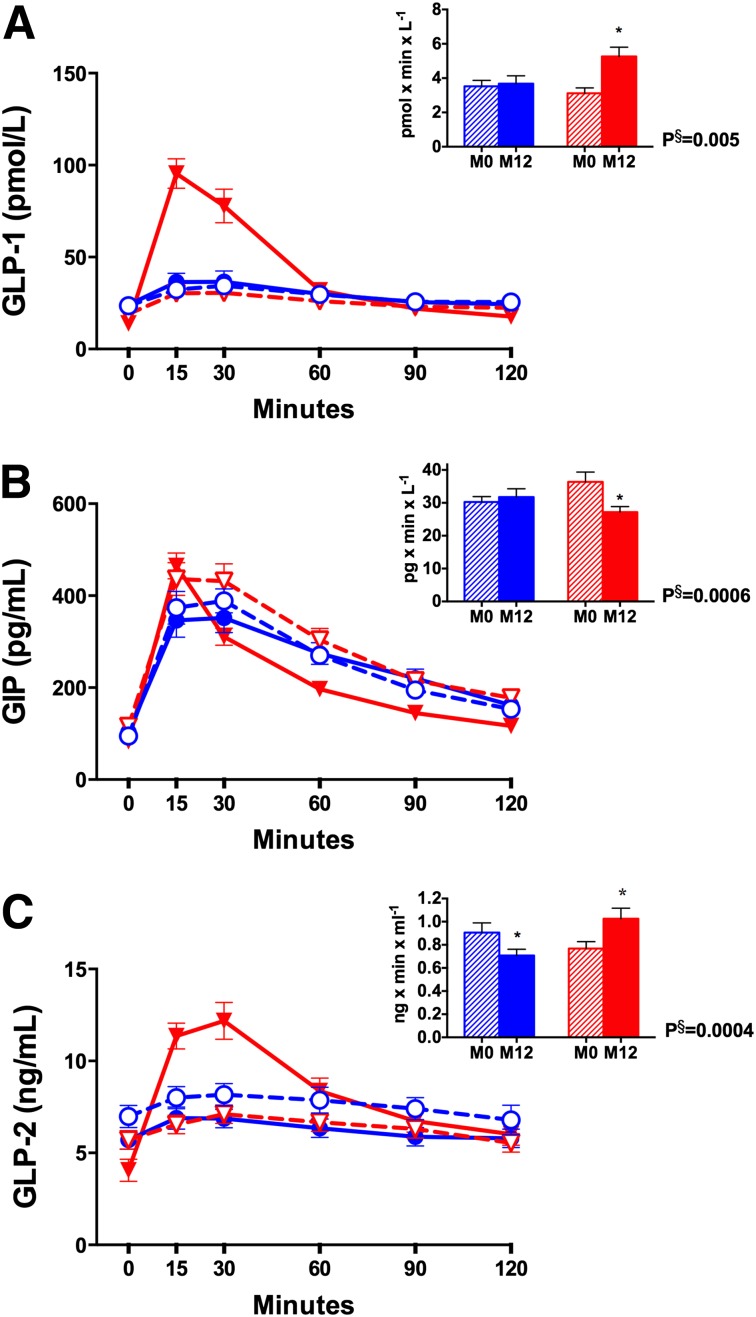
Values for GLP-1, GIP, and GLP-2 are presented in Table 1 and Fig. 2. The GLP-1 AUC increased after RYGB but not after LS/IMM. Despite an early postprandial increase in GIP levels, there was an overall significant decrease in the GIP AUC after RYGB. There was no change in GIP after LS/IMM. Peak GLP-1 or GIP levels were not associated with peak insulin or C-peptide levels (data not shown). The GLP-2 AUC decreased in LS/IMM patients but increased in RYGB patients.
Figure 2.
Fasting and postprandial changes in GLP-1 (A), GIP (B), and GLP-2 (C). Values are depicted in blue for LS/IMM and in red for RYGB. Open shapes and dashed lines denote baseline values, and closed shapes and solid lines reflect values at 1 year. Hatched bars denote baseline values, and solid bars denote values at 1 year. AUC is calculated from 0 to 120 min and is expressed ×103. M0, month 0 (baseline); M12, month 12. *P < 0.05 for within-group change. §P < 0.05 for difference in change between groups.
Correlation Analyses
Various factors were examined to determine predictors of HbA1c at 12 months (Table 2). At 12 months, weight loss was significantly correlated with HbA1c in both groups. Baseline HbA1c was predictive of HbA1c at 1 year in LS/IMM. Baseline 90-min C-peptide and greater early-phase (30 min) insulin secretion were associated with lower HbA1c at 1 year in RYGB. A higher C-peptide index was correlated with lower HbA1c for RYGB patients, and greater early postprandial insulin secretion at 1 year continued to correlate with lower HbA1c. The oDI was correlated with lower HbA1c for both LS/IMM and RYGB. BCGS correlated with HbA1c at 1 year for both groups; this association was independent of weight loss. BCGS did not correlate with weight loss, GLP-1 at 30 min, or total GLP-1 AUC (data not shown). Neither HOMA-IR nor MI were correlated with HbA1c at 1 year for either group, but higher adiponectin levels at 1 year in RYGB patients correlated with lower HbA1c. After taking into account weight loss, adiponectin was no longer predictive of HbA1c at 1 year in either group. Gut hormone levels before and after either intervention were not correlated with HbA1c.
Table 2.
Correlation between clinical and biochemical parameters and month 12 HbA1c
| IMM |
RYGB |
|||
|---|---|---|---|---|
| r | P | r | P | |
| Duration of T2DM | 0.35 | 0.05 | 0.13 | 0.49 |
| BMI M0 | 0.05 | 0.80 | −0.15 | 0.42 |
| HbA1c M0 | 0.43 | 0.01 | 0.08 | 0.68 |
| Insulin use M0 | 0.20 | 0.27 | 0.16 | 0.37 |
| C-peptide M0 | ||||
| Fasting | −0.35 | 0.05 | −0.31 | 0.09 |
| 90-min | −0.29 | 0.13 | −0.50 | 0.007 |
| % Weight loss | 0.41 | 0.02 | 0.46 | 0.008 |
| C-peptide index | ||||
| M0 | 0.07 | 0.73 | −0.35 | 0.07 |
| M12 | −0.42 | 0.02 | −0.54 | 0.003 |
| HOMA-IR | ||||
| M0 | −0.18 | 0.35 | −0.13 | 0.47 |
| M12 | 0.25 | 0.17 | 0.24 | 0.19 |
| MI | ||||
| M0 | 0.21 | 0.25 | 0.13 | 0.49 |
| M12 | −0.24 | 0.19 | −0.10 | 0.58 |
| oDI | ||||
| M0 | −0.005 | 0.98 | −0.13 | 0.52 |
| M12 | −0.52 | 0.009 | −0.69 | <0.0001 |
| BCGS | ||||
| M0 | −0.01 | 0.96 | −0.21 | 0.33 |
| M12 | −0.44 | 0.07 | −0.69 | 0.0002 |
| ISR/glucose AUC0–30 | ||||
| M0 | −0.28 | 0.18 | −0.48 | 0.01 |
| M12 | −0.32 | 0.13 | −0.71 | <0.0001 |
| GLP-1 AUC | ||||
| M0 | −0.35 | 0.09 | −0.09 | 0.67 |
| M12 | −0.08 | 0.65 | 0.06 | 0.76 |
| GLP-2 AUC | ||||
| M0 | 0.09 | 0.67 | −0.07 | 0.73 |
| M12 | 0.23 | 0.21 | 0.09 | 0.62 |
| GIP AUC | ||||
| M0 | 0.17 | 0.43 | −0.05 | 0.80 |
| M12 | −0.01 | 0.94 | 0.19 | 0.29 |
| Glucagon AUC | ||||
| M0 | 0.02 | 0.93 | −0.31 | 0.13 |
| M12 | 0.05 | 0.77 | 0.08 | 0.69 |
| Adiponectin | ||||
| M0 | 0.01 | 0.95 | 0.002 | 0.99 |
| M12 | −0.17 | 0.34 | −0.40 | 0.03 |
Clinical and biochemical parameters assessed with Spearman partial correlation, adjusting for age and sex.
AUC is calculated from 0 to 120 min, unless otherwise indicated.
M0, month 0; M12, month 12.
Discussion
We assessed pancreatic function and gut hormone changes after LS/IMM, alone or with RYGB, to determine factors associated with glycemic improvement after RYGB that may help identify patients most likely to derive the greatest glycemic benefit from surgery. As expected, fasting glucose and HbA1c improved in both groups but more so after RYGB; unexpectedly, the glucose AUC decreased similarly in both groups. We found that when insulin and gut hormone responses are compared between treatment groups, it is important to consider altered dynamics after RYGB that are likely due to accelerated transit and absorption of nutrients (12–14). Therefore, the discussion below considers both fasting and postprandial changes.
The significant decline in total insulin response after RYGB disguises a greater early postprandial peak in insulin secretion. RYGB patients experienced a rapid insulin increase early after meal ingestion, followed by a precipitous decline, whereas insulin secretion increased in LS/IMM patients and remained elevated. The early postprandial increase in insulin secretion after RYGB was significantly associated with lower HbA1c. C-peptide and insulinogenic indices both increased and were correlated with lower HbA1c after RYGB but remained unchanged after LS/IMM. Although higher insulin secretion may be the expected response to an early glycemic stimulus with altered nutrient transport, the correlation between early insulin secretion and improved HbA1c suggests that RYGB improves intrinsic β-cell glucose sensitivity.
Greater early improvement in hepatic insulin sensitivity may be partly responsible for improved insulin clearance after RYGB, as noted by others (15); however, postprandial metabolic clearance was similar in both groups. Insulin sensitivity was several-fold greater after RYGB than after LS/IMM. The MI, used as a measure of peripheral insulin sensitivity (10), was associated with weight loss in both groups but did not correlate with HbA1c after 1 year. β-Cell function (oDI) showed greater improvement after RYGB and was significantly associated with glycemia and weight loss at 1 year in both groups. BCGS, although unchanged after LS/IMM and markedly increased after RYGB at 1 year, correlated significantly with improved HbA1c for both treatment groups, even after adjusting for weight loss. Taken together, the significant association of BCGS and oDI with improved HbA1c after 1 year highlights the importance of β-cell function and sensitivity in determining glycemic control, regardless of weight loss.
Altered gut hormone secretion has been proposed as an important mechanism for improved glycemia after RYGB (2). Postprandial secretion of GLP-1 has been shown to substantially and durably increase after RYGB but not after laparoscopic adjustable gastric banding or diet-induced weight loss (16,17). Our results corroborate earlier studies demonstrating an exaggerated increase in the GLP-1 AUC after RYGB without a significant change in LS/IMM. GLP-1 levels did not correlate with HbA1c at 1 year, in contrast to other studies that have implicated GLP-1 as a predictor of remission (18). Some studies have implicated an exaggerated GLP-1 response as a mediator of improved β-cell function after RYGB (14,19), but BCGS was not associated with GLP-1 in our study. Measurements of peripheral levels of GLP-1, however, may not reflect GLP-1 receptor action (20).
The insulin-to-glucagon molar ratio in RYGB patients decreased, which is consistent with relative hyperglucagonemia after RYGB, as proposed by Camastra et al. (21). Given that glucagon and GLP-1 are both derived from preproglucagon, it is possible that relative hyperglucagonemia is due to aberrant cleavage within the intestinal L cells after RYGB and/or lack of α-cell suppression (22). GLP-2, another product of preproglucagon that is cosecreted with GLP-1 in equimolar amounts, is similarly increased after RYGB. GLP-2 directly affects bowel mucosa by increasing the absorptive surface area, which may limit malabsorption and account for how well surgery is tolerated (23). GLP-2 has also been shown to stimulate glucagon secretion (24).
HbA1c at 12 months correlated most strongly with weight loss for both intervention groups. BCGS and oDI also correlated with lower HbA1c in both groups, and the association was independent of weight loss. Before surgery, a greater C-peptide index and greater 90-min postprandial C-peptide level were predictive of lower HbA1c at 1 year, suggesting that residual β-cell function plays an important role in remission. After 1 year, these parameters and greater early insulin secretion continued to correlate with lower HbA1c in RYGB patients. C-peptide and insulinogenic indices were essentially unchanged in LS/IMM patients, consistent with continued impaired insulin secretion. For LS/IMM patients, the only baseline predictor of lower HbA1c at 1 year was HbA1c, showing that aside from weight loss, different mechanisms mediate improvement in glycemia in LS/IMM versus RYGB patients.
In summary, we have shown that improved β-cell function and BCGS contribute significantly to improved glycemia regardless of weight loss and intervention. Greater insulin secretory capacity is predictive of outcome after RYGB, which may be used to identify surgical candidates most likely to achieve the greatest glycemic benefit. Optimization of the surgical procedure to maximize weight loss in combination with targeted therapies that enhance β-cell function are likely to improve surgical outcomes.
Article Information
Acknowledgments. Study Coordinators: Joyce Schone, RD, and Nyra Wimmergren, RN, University of Minnesota, Minneapolis, MN; Heather A. Bainbridge, RD, CDN, Columbia University Medical Center, New York, NY. Expert Laboratory Technical Assistance: Irene Conwell, Columbia University Medical Center, New York, NY. Data Safety Monitoring Board Members: David Nelson, PhD, Minnesota VA Health Care System, Minneapolis, MN; Victor J. Steves, PhD, the Center for Health Research, Portland, OR; J. Michael Gonzalez-Campoy, MD, PhD, FACE, Medical Director and CEO of the Minnesota Center for Obesity, Metabolic and Endocrinology Professional Association, Eagan, MN; and Raymond Drew, MD, Abbott Northwestern Hospital, Minneapolis, MN. The investigators recognize the contributions of Stanley E. Williams, PhD, University of Minnesota. None of those named herein received compensation for those referrals.
Funding. The DSS was supported by Covidien, Mansfield, MA. This publication was supported in part by grant UL1-TR000040 and grant UL1-RR024156 to Columbia University, both from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), formerly the National Center for Research Resources; NIH DK-072011 (J.K.); discretionary funds from the Columbia University Department of Medicine (J.K.); Endocrine Fellows Foundation (K.T.N.); and T32-DK-007271-33S1 Sup (K.T.N.).
The sponsoring agency had no role in the collection, management, analysis, and interpretation of the study data, and had no part in the preparation of the manuscript. The sponsor was allowed to review the manuscript prior to submission but had no role in the decision to submit the manuscript for publication.
Duality of Interest. C.J.B. reports receiving grant support from Covidien and personal support for consultancy from EnteroMedics Inc. A.V. reports receiving consulting support from Sanofi, Roche, and Novartis, institutional consulting support from Merck, and institutional grant support from Covidien, Daiichi-Sankyo, Merck, and GI Dynamics. L.A. reports receiving institutional grant support from Covidien. J.P.B. reports receiving institutional support for the Look AHEAD study. M.B. reports receiving institutional grant support from Covidien, personal consulting support from Geshon Lehmar, and personal support for malpractice review. J.E.C. reports receiving institutional and personal grant support from Covidien. A.T. reports receiving salary support from Covidien for the DSS and supplemental salary support from Minnesota Obesity Center. S.I. serves as an advisory board member for Novo Nordisk, USGI, and Medica, consults for Metamodix Inc., and receives grant support from Covidien, EnteroMedics, and ReShape Medical. J.K. reports receiving institutional grant support from Covidien and personal support for serving on the Takeda Speaker Bureau and consulting for L.E.K. Consulting. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.T.N., C.J.B., L.A., J.P.B., M.B., W.B.I., S.I., and J.K. contributed to patient care. K.T.N., C.J.B., A.V., Q.W., J.E.C., A.T., and J.K. interpreted data. K.T.N., C.J.B., A.V., Q.W., J.E.C., A.T., S.I., and J.K. drafted and reviewed the manuscript. C.J.B., J.P.B., J.E.C., W.B.I., A.T., S.I., and J.K. contributed to the study concept and design. J.K. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. Q.W., J.E.C., A.T., and J.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster (Abstract #T387) at the Annual Scientific Meeting of the Obesity Society, Atlanta, GA, 12–16 November 2013.
References
- 1.Sjöström L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(Suppl. 7):S93–S97 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep 2014;14:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association . Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care 2015;38(Suppl.):S4. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Zimmet P, Alberti KG, Rubino F; International Diabetes Federation Taskforce on Epidemiology and Prevention . Bariatric surgery: an IDF statement for obese type 2 diabetes. Arq Bras Endocrinol Metabol 2011;55:367–382 [DOI] [PubMed] [Google Scholar]
- 5.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AJ, Bainbridge HA, Schone JL, et al. Recruitment and screening for a randomized trial investigating Roux-en-Y gastric bypass versus intensive medical management for treatment of type 2 diabetes. Obes Surg 2014;24:1875–1880 [DOI] [PubMed] [Google Scholar]
- 7.Polonsky KS, Licinio-Paixao J, Given BD, et al. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 1986;77:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 2014;22:2003–2009 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Upregulation of intestinal glucose transporters after Roux-en-Y gastric bypass to prevent carbohydrate malabsorption. Obesity (Silver Spring) 2014;22:2164–2171 [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013;62:3044–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bojsen-Møller KN, Dirksen C, Jørgensen NB, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 2014;63:1725–1737 [DOI] [PubMed] [Google Scholar]
- 16.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutia R, Brakoniecki K, Bunker P, et al. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 2014;63:1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habegger KM, Heppner KM, Amburgy SE, et al. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes 2014;63:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camastra S, Muscelli E, Gastaldelli A, et al. Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes 2013;62:3709–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia 1983;25:396–399 [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ. Glucagon-like peptide 2. J Clin Endocrinol Metab 2001;86:1759–1764 [DOI] [PubMed] [Google Scholar]
- 24.Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab 2011;300:E1038–E1046 [DOI] [PubMed] [Google Scholar]