Abstract
Objective To examine the efficacy of an enhanced intervention (EI) compared to standard care (SC) in increasing daily water intake and fluid goal adherence in children seeking treatment for retentive encopresis. Methods Changes in beverage intake patterns and fluid adherence were examined by comparing 7-week diet diary data collected during participation in the EI to achieved data for families who had previously completed the SC. Results Compared to children in SC (n = 19), children in the EI (n = 18) demonstrated a significantly greater increase in daily water intake from baseline to the conclusion of treatment ( p ≤ .001), and were four and six times more likely to meet fluid targets in Phases 1 (Weeks 3–4) and 2 (Weeks 5–6) of fluid intervention, respectively (both p ≤ .001). Conclusions Enhanced education and behavioral strategies were efficacious in increasing children’s intake of water and improving fluid adherence. Future research should replicate the findings in a prospective randomized clinical trial to discern their effectiveness.
Keywords: adherence, encopresis, nutrition
Previously, we found that children participating in an empirically supported group behavioral intervention for the treatment of retentive encopresis (Opipari, Miller, Streisand, & Stark, 1999) demonstrated poor adherence (<50%) to prescribed fluid goals and relied heavily on juice in efforts to meet these targets (Kuhl, Felt, & Patton, 2009). Children receiving this treatment may be at risk for ongoing or worsening symptoms of constipation if they fail to meet fluid targets but are adherent to recommendations to increase their daily fiber intake, especially as stool softening medications are weaned (Felt, Brown, Van Harrison, Kochhar, Patten, 2008; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, 2006). In addition, 46% of our sample exceeded the American Academy of Pediatrics’ (AAP) age-based guidelines for daily juice intake (AAP, 2001) during the last week of treatment. Juice consumption in excess of these guidelines places children at heightened risk for developing dental carries or becoming overweight. Collectively, these two findings indicated that modifications to the preexisting protocol should be made to improve fluid goal adherence and children’s consumption of healthier fluid choices as they increased their fluid intake. The purpose of this study was to examine the efficacy of an educational and behavioral enhancement (enhanced intervention, EI) in increasing fluid intake for children participating in the group behavioral intervention for the treatment of retentive encopresis (Opipari et al., 1999).
Fluid intake is an understudied area within the pediatric literature, despite the commonality of fluid recommendations in the management of pediatric illnesses such as chronic constipation, migraine headache, sickle cell disease, and obesity. Families participating in our program anecdotally reported that children had difficulty meeting their prescribed fluid targets because they often denied thirst and refused to drink unless provided with preferred beverages such as juice. Identified barriers to food-based recommendations for young children with chronic health conditions, such as satiation and parental use of ineffective behavior management strategies (Patton, Dolan, & Powers, 2006; Stark & Powers, 2005), are likely applicable to understanding why children have difficulty increasing and modifying their fluid intake. Interventions combining educational and behavior modification strategies have been highly effective in addressing these barriers and improving adherence to nutrition-based recommendations for young children (Stark, 2003), so a similar treatment package might be effective in improving adherence to fluid-based recommendations.
This study reports on the efficacy of a fluid-specific educational and behavioral enhancement (EI) to the 7-week, empirically supported group behavioral intervention protocol designed by Stark and colleagues (Opipari et al., 1999) in a sample of treatment-seeking children with retentive encopresis. The fluid intervention targeted increasing clear fluid goal adherence and children’s daily intake of water. We tested the following hypotheses: (a) children receiving the EI would demonstrate a significantly greater increase in their daily water intake from baseline (Weeks 1 and 2) to the conclusion of the first fluid phase (Week 4), and from the baseline phase to the conclusion of the second fluid phase (Week 6) compared to children who received the original group behavioral intervention (standard care; SC); (b) participation in the EI would significantly increase the odds of children meeting prescribed daily clear fluid goals in fluid Phase 1 and in fluid Phase 2 compared to participation in the SC; and (c) participation in the EI would significantly decrease the odds of children exceeding the AAP age-based guidelines for daily juice intake (AAP, 2001) during the active phases of fluid intervention (Weeks 3–6).
Methods
Recruitment and Participants
This study was conducted at a large, Midwestern university medical center and was approved by the Institutional Review Board (IRB). Families were referred for treatment within our clinic either by their primary care physician, a pediatric gastroenterologist, or they were self-referred. Inclusion criteria were child aged between 4 and 12 years and a diagnosis of retentive encopresis. Families were excluded if the child had a diagnosis of developmental delay, the child or parents had a history of severe psychopathology that required hospitalization, and/or the family had fewer than 5 days of diet diary data for the first or last weeks of group treatment. In addition, we excluded entire treatment groups in situations when we had to deviate from our standard 7-week schedule due to weather or scheduling problems. The SC group consisted of archived daily diet diary data for families who participated in the group behavioral intervention from 2005 to 2007. Documentation of a waiver of informed consent for use of this data was obtained from the IRB, as daily diet diaries were collected as part of treatment. Children in the EI were prospectively recruited immediately following their diagnostic intake appointment in our clinic between 2007 and 2008. Families interested in participating in this study spoke to a research assistant who completed the informed consent process.
Forty-three children who completed treatment from 2005 to 2007 (SC) and 25 children who completed treatment from 2007 to 2008 (EI) were eligible for participation in this study. Within the SC group, three children were excluded due to missing data and 21 children were excluded because they came from treatment groups that deviated from the 7-week schedule. For the EI group, one child was excluded because the family chose not to complete diet diaries during treatment and six children were excluded because they came from treatment groups that did not meet the 7-week criterion. The final sample included 37 children: 18 in the EI and 19 in the SC. The EI was 61% males, 72% whites, had a mean age of 6.22 years (SD ± 2.44), had experienced symptoms of encopresis for a mean of 3.55 years (SD ± 2.13), and had a mean body mass index (BMI) of 16.89 (SD ± 2.52). The SC was 68% males, 79% whites, had a mean age of 6.47 years (SD ± 2.14), had experienced symptoms of encopresis for a mean of 3.29 years (SD ± 2.31), and had a mean BMI of 17.13 (SD ± 1.72). Treatment groups did not differ significantly on any demographic variables.
Measures
Diet Recording
During the first group treatment session, parents in both groups were trained in diet monitoring. Parents were asked to document all foods and beverages consumed by children in standardized paper diet diaries (Stark, Bowen, Tyc, Evans, & Passero, 1990) for each day of group treatment and return their completed diet diaries at each treatment session. Feedback on their self-monitoring efforts was provided, including graphs that depicted children’s daily and average clear fluid and fiber intake. Once children received their first dietary goals (Week 3), feedback regarding strategies for improving dietary adherence was also provided.
Group Behavioral Intervention
Families in both the SC and the EI completed six 1-hr group sessions over 7 weeks following the group treatment program for encopresis designed by Stark and colleagues (Opipari et al., 1999). Due to developmental differences and age-specific school and social consequences of retentive encopresis, families were placed into either a younger (ages 4–6) or older (ages 7–12) child group. Within each group, parents and children were seen separately but simultaneously. Parent groups were run by a Developmental–Behavioral Pediatrician and a Pediatric Psychologist or a Psychology Post-Doctoral Fellow. Child groups were run by a Post-Doctoral Fellow or a master’s level graduate student. Session topics included the physiology of retentive encopresis, medication, fiber and clear fluids, toilet sits, and strategies for maintaining treatment gains (see Opipari et al., 1999 for more detail). Children received weekly goals specific to session topics and could earn prizes at subsequent group sessions if their sticker charts revealed that they had met at least 75% of their weekly goals.
Fluid intervention for both groups included teaching parents how to differentiate clear fluids (all nondairy, noncaffeinated fluids) and prescription of clear fluid goals using a changing criterion design, with each goal being held for 2 weeks. More specifically, children received their first clear fluid goal (in fluid ounces) during Session 3, which was equivalent to twice the child’s age-based fiber goal: [age + 5(grams of fiber)] × 2 (Felt et al., 2008). During Session 5, clear fluid goals were increased to twice children’s new fiber goal of [age + 10 (grams of fiber)] × 2.
Fluid Enhancements (EI only)
Fluid-specific modifications occurred over the course of two sessions (Sessions 3 and 5) and in three broad areas: enhanced fluid education, prescription of water goals, and teaching parents how to apply child behavior management techniques to increase their child’s daily water intake. During Session 3, parents were provided with the AAP age-based guidelines for children’s juice intake (AAP, 2001) and were taught how to apply behavioral strategies (differential attention, rules, modeling, stimulus generalization, and liquid fading) to increase their child’s water intake. Children learned the same fluid education but it was modified for their developmental level and taught through creative games and activities. In addition to daily clear fluid goals, children in the EI received daily meal-based water goals (4 fluid ounces per meal for children aged 4–6 years; 6 fluid ounces for children aged 7–12 years) and a daily water bottle goal (8 fluid ounces for all children). Children were provided with a 16-ounce Nalgene® water bottle to assist them with meeting their daily water bottle goal. During Session 5, meal-based water goals were increased to 6 fluid ounces per meal for younger children and 8 fluid ounces per meal for older children. Water bottle goals remained the same for younger children but were increased to 16 fluid ounces for older children. Parents and children were provided with several low-calorie, sugar-free drink choices and water recipes to increase the variety of healthy water choices for children given children's larger water goals.
As water is a clear fluid, values for water goals were devised to facilitate children’s adherence to their clear fluid goals. More specifically, children’s water goals were set so that 100% adherence to daily water goals would equate with meeting or being slightly below their prescribed daily clear fluid goals. Constructing goals in this way also increased the possibility that a large portion of children’s total daily fluid intake would consist of water. For example, on a given day in Fluid Phase 1, a 6-year-old child who met all prescribed daily water goals (20 fluid ounces) would only need to consume 2 additional ounces of a clear fluid to meet his daily clear fluid goal (22 fluid ounces). If the same 6-year-old child met all of his daily water goals in Fluid Phase 2 (26 fluid ounces), he would need to consume only 6 more ounces of a clear fluid of choice to meet his daily clear fluid goal (32 fluid ounces).
Treatment Integrity
All clinic sessions of the EI were videotaped and evaluated by trained coders to assess treatment integrity to the fluid enhancements only. Video-taping sessions was not part of standard care for children completing group treatment at our clinic during 2005–2007 (SC group). Coding of video-taped sessions revealed 83–100% adherence to the EI treatment manual and inter-rater reliability was 93% (κ = .92).
Data Entry and Analysis
Diet diary data were analyzed using the Food Processor SQL program (2005 version, ESHA Research). Nutrition variables of interest were entered with demographic variables into a panel data set (Hsiao, 2003) and all analyses were conduced using SAS (Statistical Analysis Software Version 9.1). Data were grouped into the following three phases for analysis purposes: baseline (Weeks 1–2), fluid Phase 1 (Weeks 3–4), and fluid Phase 2 (Weeks 5–6).
Defining Adherence
Adherence was measured dichotomously on a daily basis for all adherence variables. For clear fluid goals (SC and EI) and water goals (EI only), children who met or surpassed their prescribed daily goals were labeled adherent and children who did not achieve their prescribed daily goals were labeled nonadherent. For the AAP juice guidelines (AAP, 2001), children were labeled adherent if their daily juice intake was equal to or less than the AAP guidelines for juice intake (6 fluid ounces for children aged ≤6 years and 12 fluid ounces for children aged 7–12 years) and labeled nonadherent if their juice intake exceeded the recommended values for their age.
Statistical Analyses
Only significant fluid [water, juice, sugar-sweetened beverages (SSBs), milk, and soda] and nutrient (calories and fiber) covariates were entered into models used to test our primary hypotheses; demographic variables were not included as treatment groups did not differ significantly on these variables at baseline. Bonferroni corrections were applied to all analyses used to test our hypotheses and statistical significance was achieved for p ≤ .01. To test Hypothesis 1, we used a mixed-effects model for analyzing change over time (PROC MIXED procedure in SAS) with three random effects, which were allowed to correlate with one another: baseline, baseline–phase 1 change, and baseline–phase 2 change. Fixed effects of primary interest were baseline, group, baseline–phase 1 change, baseline–phase 1 change by group interaction, baseline–phase 2 change, and baseline–phase 2 change by group interaction. Within-individual model residuals were assumed constant and uncorrelated over time.
The PROC GENMOD procedure in SAS was used to examine Hypotheses 2 and 3. Specifically, logistic regressions were carried out using this procedure, because the outcomes in Hypotheses 2 and 3 were all dichotomous (e.g., adherent and non-adherent). All logistic regressions carried out in PROC GENMOD were based on a generalized estimating equations approach where an exchangeable working correlation structure was employed to account for within-subject correlation in the analyses. The fixed effect of primary interest in these models was group status. For Hypothesis 2, two separate analyses were performed (one for each of the fluid phases) given the difference in children’s clear fluid goals for Fluid Phases 1 and 2. Covariates included in the models used to test Hypothesis 2 included treatment group (EI vs. SC) and children’s daily intake of milk and calories. For Hypothesis 3, between-group differences in adherence to the AAP age-based daily juice guidelines was examined across the fluid intervention as a whole (Weeks 3–6) and by age group to reflect the two different age groups described in the AAP guidelines for children’s daily juice intake. For younger children, covariates included in the model to test adherence to AAP guidelines were treatment group and children’s daily intake of soda, water, and calories. For older children, covariates included in the model to test adherence to AAP guidelines were treatment group and children’s daily intake of soda, milk, and calories.
In addition to testing our hypotheses, we also conducted two exploratory analyses to further evaluate changes in children’s fluid intake. First, for prescribed water goals (EI only), group percent adherence was calculated for each individual water goal by dividing the number of goals met for the group as a whole (numerator) by the total number of possible water goals for the group as a whole (denominator). Adherence was examined for the fluid intervention as a whole (Weeks 3–6) and by individual fluid phase. Second, the same PROC MIXED procedure used to examine Hypothesis 1 was applied to evaluate between-group differences for changes in children’s mean daily intake of total clear fluid and their intake of milk, SSBs, and soda from baseline to the conclusion of Fluid Phase 1 and from the baseline phase to the conclusion of Fluid Phase 2.
Results
Preliminary Analyses
Between-group differences in mean daily intake for each type of fluid assessed at each fluid phase are presented in Figure 1. Group means for the main-dependent variables of interest are reported by phase in Table I. Data regarding bowel movement functioning were available for 16 participants in the SC and all participants in the EI. Treatment groups did not differ significantly in the frequency of soiling accidents per week during the first (both groups M = 6, SD = 8) or final week of treatment (SC M = 6, SD = 12; EI M = 4, SD = 6) but the observed decrease in soiling accidents was statistically significant for children in the EI (t17 = −2.29, p ≤ .05). Treatment groups also did not differ significantly in the frequency of bowel movements in the toilet per week during the first (SC M = 5, SD = 3; EI M = 6, SD = 5) or final week of treatment (SC M = 7, SD = 4; EI M = 9, SD = 7), but there was a trend that this increase was significant for children in the EI (t17 = 1.85, p = 08).
Figure 1.
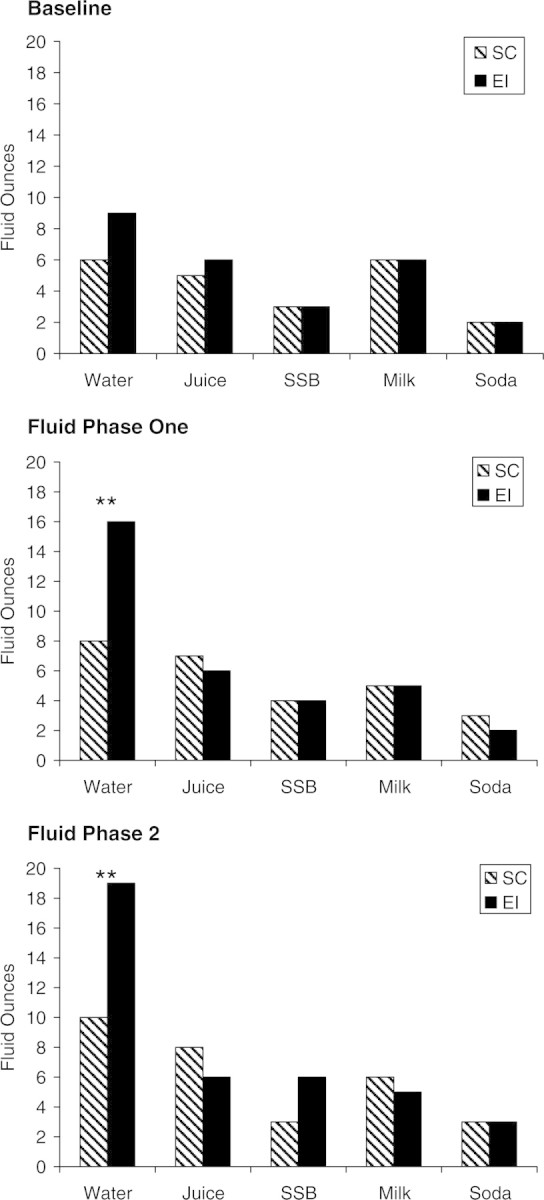
Between-group comparison of mean (model-based estimates) daily intake for all fluid types by fluid phase. **p ≤ .001. SSB, Sugar-sweetened beverage.
Table I.
Mean Values for Main Dependent Variables of Interesta
| Baseline | Fluid Phase 1 | Fluid Phase 2 | |
|---|---|---|---|
| Daily water intakeb | |||
| EI | 9 | 17 | 20 |
| SC | 7 | 9 | 10 |
| Daily CF intakeb | |||
| EI | 18 | 26 | 32 |
| SC | 16 | 20 | 22 |
| Daily juice intakeb | |||
| EI | 6 | 5 | 5 |
| SC | 5 | 7 | 8 |
| Group percentage of CF goal adherence | |||
| EI | – | 70 | 63 |
| SC | – | 46 | 29 |
aMean values are not based on model estimates. Standard deviations are not included because they would reflect between- and within-group variance given the time-series and cross-sectional nature of our design, and thus would be an inaccurate representation of the variance.
bValues reported in fluid ounces.
CF, clear fluid.
Water Intake
Consistent with Hypothesis 1, EI children demonstrated a significantly greater increase in their mean daily water intake from Baseline to the conclusion of Phase 1 [t1,326= 2.54, p ≤ .01, β = 4.84, confidence interval (CI) = 1.12–8.58; d = .97] and from Baseline to the conclusion of Fluid Phase 2 (t1,326= 3.50, p ≤ .001, β = 7.38, CI = 3.24–11.51; d = .95) compared to SC children.
Clear Fluid Adherence
Consistent with Hypothesis 2, participation in the EI increased the odds of children meeting their clear fluid goals by 3.71 (p ≤ .01) during Fluid Phase 1 and by 6.01 (p ≤ .001) during Fluid Phase 2 compared to participation in the SC. Children in the EI demonstrated a significantly greater increase in their overall clear fluid intake from baseline to the conclusion of Fluid Phase 2 (t1,329 = 2.93, p ≤ .05, β = 5.52, CI = 0.86–10.18; d = .89) compared to children in the SC. No additional significant differences specific to clear fluid intake were observed.
Age-based Juice Guidelines
Hypothesis 3 was partially supported. Participation in the EI significantly decreased the odds of exceeding the daily juice guidelines for older children only (odds ratio = 0.22, p ≤ .001). However, children in the EI demonstrated a significantly greater decrease in their juice intake from baseline to Fluid Phase 1 (t1,327= −1.95, p ≤ .05, β = 2.52, CI = −2.99 to 0.01; d = .96) and from baseline to Fluid Phase 2 (t1,327 = −2.01, p ≤ .05, β = 2.64, CI = −4.48 to −0.06; d = .77) compared to children in the SC.
Water Goal Adherence (EI only)
Group mean percent adherence for water goals during Fluid Phase 1 was 39% for breakfast, 32% for lunch, 45% for dinner, and 73% for water bottles. For Fluid Phase 2, group mean percent adherence was 38% for breakfast, 39% for lunch, 47% for dinner, and 79% for water bottles.
Discussion
This study is the first to systematically target increasing daily water intake and adherence to fluid goals for children with retentive encopresis. We found that children in the EI made significantly greater increases in their daily water intake and were four to six times more likely to meet their clear fluid goals than children in the SC. While the difference was not statistically significant, mean daily intake of juice was lower for children in the EI compared to children in the SC.
Several important clinical and research implications emerge from our findings. First, we were able to modify children’s daily fluid intake to include more water. While this change was observed for children in both treatment conditions, it was significantly greater for children in the EI. Our findings provide further support for the efficacy of combined enhanced nutritional education and behavioral strategies in modifying dietary intake and improving dietary adherence (Kahana, Frazier, & Drotar, 2008). Second, by increasing children’s water intake, we were able to improve the likelihood that they would meet clear fluid targets, and this has the potential to improve the effectiveness of the group behavioral treatment as a whole. Future research is needed to examine this question by comparing the effectiveness of the EI to the original group behavioral treatment protocol (Opipari et al., 1999) in a prospective randomized controlled trial. Establishment of clear fluid adherence also now provides a platform for empirical investigation of the proposed relationship between increased fluid intake and decreased symptoms of constipation. Third, many studies within the encopresis literature make reference to including fluid recommendations within multi-component interventions for this disorder (McGrath, Mellon, & Murphy, 2000) but few report specific fluid targets or adherence to fluid recommendations. The current study addresses these gaps in the literature and provides direction to health care providers who wish to include fluid recommendations in their prescriptions for children receiving treatment for this condition.
Fourth, the fact that children in our sample were able to improve fluid adherence while concurrently making other lifestyle (e.g., adherence to toilet sitting schedules) and nutritional (increasing daily fiber intake) changes suggests that a similar approach could be effective for other pediatric illness groups where fluid recommendations constitute one component within a multi-component treatment regimen. When the fluid-specific elements are extracted from the intervention as a whole, the result is a problem-specific, time-limited, low-cost (less than 12 dollars per person for supplies) intervention. In addition, anecdotally we can report that children demonstrated a high level of engagement during fluid-specific games and activities and appeared excited when telling parents what they learned, which suggests they found the fluid enhancements to be fun. The fluid-specific elements of the EI might be easily adapted for use in outpatient psychotherapy or medical care visits and could be recommended to parents by physicians, dieticians, and other members of health care teams with appropriate training.
Finally, our findings suggest the importance of examining adherence in other pediatric populations where fluid recommendations are thought to be an integral part of care. For example, no published data are available for children being treated for migraine or sickle cell disease who are asked to increase their fluid intake to prevent pain episodes. The poor adherence and unhealthy trends observed for children in the SC within our sample suggest a need for new research exploring adherence to fluid recommendations and the types of beverages children and adolescents consume in their adherence efforts in other pediatric populations where fluid intake is an important component of daily care. In addition, although published data exist regarding intake of SSBs and soda for children who are overweight and obese (Malik, Schulze, & Hu, 2006), little is known about water intake or changes these children make to their fluid intake when instructed to eliminate high-calorie beverages from their diet. Addressing this gap has clinical relevance because failure to adhere to these recommendations could thwart weight loss efforts or lead to dehydration if children do not replace SSBs with low- to no-calorie fluids such as water. Future research should thus examine fluid adherence within these and other applicable pediatric populations and explore the efficacy of the EI as appropriate.
The efficacy of the EI was explored within a clinical context and thus aspects of our methodology may pose challenges when generalizing our findings. This study was not controlled and participants were not randomly assigned to treatment groups. Our decision to use archived data for the control group excludes us from ruling out the potential contribution that factors such as changes in the types of fluids available for purchase, marketing, or general nutrition knowledge may have played in our results. Our small sample size also poses limitations with respect to interpretation of the significance of our findings. Thus, the next step would be to examine the effectiveness of the EI within the context of a larger prospective randomized clinical trial. In addition, while our sample is highly representative of the population served at our medical center, our findings may not be applicable to treatment facilities where families are from different ethnic and sociodemographic backgrounds or where a greater percentage of families are without private insurance.
Despite our efforts to increase the reliability of diet diary data specific to fluid intake, parental anecdotal reports suggest children’s actual fluid intake was likely still underreported, especially during times and settings where parents were not present (e.g., school). Reliability of self-reported data might also have been influenced by social desirability and recency effects. In addition, EI parents may have been more accurate in their recording of fluid data compared to SC parents due to the greater emphasis on fluid in the EI. Future research should continue to explore methodologies for improving measurement of fluid intake. For example, the format of diet diaries could be modified to include pictures of common fluid vessels served to children (e.g., milk cartons and juice boxes; Cowbrough & Llyod, 2003; Domel, Thompson, Baranowski, & Smith, 1994) or converted to an electronic format. Development of an electronic assessment tool, similar to the Medication Event Monitoring System for pill bottles or the MDILog monitors for asthma inhalers, would also provide a more objective measure of children’s adherence to daily water bottle goals.
Conclusion
We found that providing enhanced fluid education and behavioral strategies was efficacious in increasing daily water intake and fluid goal adherence for young children who received a group behavioral treatment for retentive encopresis. The qualitative changes in fluid intake for children in the EI reveal that children can increase their fluid intake with healthy choices. In addition, demonstration of fluid adherence is an important first step in examining the relationship between fluid intake and symptoms of constipation. Adherence to fluid recommendations is an area that is grossly underexamined within the pediatric literature at large, and our study reveals the importance of continuing to evaluate fluid adherence as well as a need for improved methods to measure children’s fluid intake.
Funding
This study was funded by grant support from the Blue Cross Blue Shield Foundation of Michigan (Award #1263.SAP).
Conflict of interest: None declared.
Acknowledgments
We would like to thank Fisher Scientific, Inc. for their generous donations of the Nalgene water bottles that were provided to children participating in this study. We would like to thank all of the families who so diligently kept diet diaries every day of their treatment. In addition, we would like to thank Dawn Dore-Stites, PhD, My Lien, PhD, Katie Rosen, Emily Delmott, Thomas Stimpson, Amanda Ryan, and Caryn Kosteva for their help with treatment delivery, data entry, and data analysis. Elizabeth S. Kuhl, PhD, is now a pediatric psychology fellow at Cincinnati Children's Hospital Medical Center.
References
- American Academy of Pediatrics Committee on Nutrition. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210–1214. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- Cowbrough K, Llyod H. A measurement and comparison of the fluid intake in people with and without back pain. Journal of Human Nutrition and Dietetics. 2003;16:403–409. doi: 10.1046/j.1365-277x.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- Domel SB, Thompson WO, Baranowski T, Smith AF. How children remember what they have eaten. Journal of the American Dietetic Association. 1994;94:1267–1272. doi: 10.1016/0002-8223(94)92458-9. [DOI] [PubMed] [Google Scholar]
- Felt BT, Brown PI, Van Harrison R, Kochhar PK, Patton SR, Marcus SM, Teitelbaum DH. University of Michigan Health System Guidelines for Clinical Care: Functional constipation and soiling in children. Retrieved from http://cme.med.umich.edu/pdf/guideline/peds08.pdf. [Google Scholar]
- Hsiao CH. Analysis of panel data. Cambridge; New York: Cambridge University Press; 2003. [Google Scholar]
- Kahana S, Frazier T, Drotar D. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology. 2008;33:590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- Kuhl ES, Felt BT, Patton SR. Brief report: Adherence to fluid recommendations in children receiving treatment for retentive encopresis. Journal of Pediatric Psychology. 2009;34:1165–1169. doi: 10.1093/jpepsy/jsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: A systematic review. American Journal of Clinical Nutrition. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath ML, Mellon MW, Murphy L. Empirically supported treatments in pediatric psychology: constipation and encopresis. Journal of Pediatric Psychology. 2000;25:225–254. doi: 10.1093/jpepsy/25.4.225. [DOI] [PubMed] [Google Scholar]
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Clinical Practice Guideline: Evaluation and treatment of infants and children: Recommendations of the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition. 2006;43:e1–e13. doi: 10.1097/01.mpg.0000233159.97667.c3. [DOI] [PubMed] [Google Scholar]
- Opipari L, Miller D, Streisand R, Stark L. Retentive encopresis. In: Shaefer CE, editor. Short-term psychotherapy groups for children: Adapting group processes for specific problems. Northvale: NJ: Jason Aronson; 1999. [Google Scholar]
- Patton SR, Dolan LM, Powers SW. Mealtime interactions relate to dietary adherence and glycemic control in young children with type 1 diabetes. Diabetes Care. 2006;29:1002–1006. doi: 10.2337/diacare.2951002. [DOI] [PubMed] [Google Scholar]
- Stark LJ. Adherence to diet in chronic conditions: The example of cystic fibrosis. In: Drotar D, editor. Promoting adherence to medical treatment in childhood chronic illness: Concepts, methods, and interventions. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 409–427. [Google Scholar]
- Stark LJ, Bowen AM, Tyc VL, Evans S, Passero AM. A behavioral approach to increasing caloric consumption in children with cystic fibrosis. Journal of Pediatric Psychology. 1990;15:309–326. doi: 10.1093/jpepsy/15.3.309. [DOI] [PubMed] [Google Scholar]
- Stark LJ, Powers SW. Behavioral aspects of nutrition in children with cystic fibrosis. Current Opinion in Pulmonary Medicine. 2005;11:539–542. doi: 10.1097/01.mcp.0000183051.18611.e4. [DOI] [PubMed] [Google Scholar]


