The cartilage regeneration potential of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) with a hyaluronic acid (HA) hydrogel composite has shown remarkable results in small animal models. A minipig model was used to confirm the consistent regenerative potential. This evidence of consistent cartilage regeneration using composites of hUCB-MSCs and HA hydrogel in a large animal model could be a stepping stone to a human clinical trial in the future.
Keywords: Cartilage, Regeneration, Human umbilical cord blood, Mesenchymal stem cell, Hyaluronic acid
Abstract
The cartilage regeneration potential of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) with a hyaluronic acid (HA) hydrogel composite has shown remarkable results in rat and rabbit models. The purpose of the present study was to confirm the consistent regenerative potential in a pig model using three different cell lines. A full-thickness chondral injury was intentionally created in the trochlear groove of each knee in 6 minipigs. Three weeks later, an osteochondral defect, 5 mm wide by 10 mm deep, was created, followed by an 8-mm-wide and 5-mm-deep reaming. A mixture (1.5 ml) of hUCB-MSCs (0.5 × 107 cells per milliliter) and 4% HA hydrogel composite was then transplanted into the defect on the right knee. Each cell line was used in two minipigs. The osteochondral defect created in the same manner on the left knee was untreated to act as the control. At 12 weeks postoperatively, the pigs were sacrificed, and the degree of subsequent cartilage regeneration was evaluated by gross and histological analysis. The transplanted knee resulted in superior and more complete hyaline cartilage regeneration compared with the control knee. The cellular characteristics (e.g., cellular proliferation and chondrogenic differentiation capacity) of the hUCB-MSCs influenced the degree of cartilage regeneration potential. This evidence of consistent cartilage regeneration using composites of hUCB-MSCs and HA hydrogel in a large animal model could be a stepping stone to a human clinical trial in the future.
Significance
To date, several studies have investigated the chondrogenic potential of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs); however, the preclinical studies are still limited in numbers with various results. In parallel, in the past several years, the cartilage regeneration potential of hUCB-MSCs with a hyaluronic acid (HA) hydrogel composite have been investigated and remarkable results in rat and rabbit models have been attained. (These experimental results are currently in preparation for publication.) Before applying the cartilage regeneration technique in a human clinical trial, it seemed necessary to confirm the consistent result in a larger animal model. At 12 weeks postoperatively, the minipigs were sacrificed, and the degree of subsequent cartilage regeneration was evaluated by gross and histological analysis. The transplanted knee resulted in superior and more complete hyaline cartilage regeneration compared with the control knee. This evidence of consistent cartilage regeneration with composites of hUCB-MSCs and HA hydrogel in a large animal model could be a stepping stone to a human clinical trial in the future.
Introduction
The ultimate goal in the treatment of cartilage injury and osteoarthritis is to achieve improved articular cartilage repair and to eliminate or significantly reduce pain and inflammation by restoring mechanically functional cartilage tissue [1]. Thus, cartilage tissue engineering has evolved as a method to restore functionally competent hyaline cartilage, similar to that in the native articular cartilage. In particular, in the past two decades, mesenchymal stem cells (MSCs) have offered great promise for cartilage tissue engineering by an understanding of their properties, including freedom from immune or inflammatory responses [2], donor independence [3], easy duplication in vitro [4], and homing capability [4]. Among the various stem cell sources available, human umbilical cord blood-derived MSCs (hUCB-MSCs) have gained increasing attention, because they are easy to obtain and store. They also do not require a close human leukocyte antigen (HLA) match owing to the naïve nature of a newborn’s immune system [5, 6].
To date, several researchers have investigated the chondrogenic potential of hUCB-MSCs; however, the preclinical studies have been limited and have yielded various results [7–11]. In the past several years, we have been investigating the cartilage regeneration potential of hUCB-MSCs, with a hyaluronic acid (HA) hydrogel composite, and attained remarkable results in rat and rabbit models [12, 13]. (The experimental results are currently in preparation for submission and publication.) Before applying the cartilage regeneration technique in a human clinical trial, it seemed necessary to confirm that the results were consistent in a larger animal model. The pig genome is very similar to the human genome [14, 15]. Thus, the pig has been increasingly used in biomedical research for studies of the spectrum of human diseases, including xenotransplantation. Therefore, the purpose of the present study was to investigate whether hUCB-MSCs with a HA hydrogel composite would show consistent regenerative potential in a minipig model.
Materials and Methods
Preparation for hUCB-MSCs and HA Hydrogel Composite
hUCB was collected from the umbilical veins after neonatal delivery by an independent cord blood bank. The pregnant mothers provided informed consent, just as with previous reports [12, 13]. The isolation and cultivation of the MSCs from the UCB were performed according to the published method [16] and donated for the present animal study. Mononuclear cells were isolated from hUCB by density gradient centrifugation at 550g for 30 minutes in a Ficoll Hypaque (density, 1.077 g/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). The separated mononuclear cells were then plated in an α-minimum essential medium (α-MEM; Gibco BRL, Carlsbad, CA, http://www.lifetechnologies.com) supplemented with 15% fetal bovine serum (FBS; HyClone, GE Healthcare Life Sciences, Logan, UT, http://www.gelifesciences.com), and maintained at 37°C in a humidified atmosphere, containing 5% CO2 with a change of culture medium, twice a week. Approximately 2 weeks later, fibroblast-like adherent cells were observed. When the monolayer of MSC colonies reached 80% confluence, the cells were trypsinized (0.25% trypsin; HyClone, GE Healthcare Life Sciences), washed, and resuspended in a culture medium (α-MEM supplemented with 10% FBS). A total of 3 hUCB-MSC lines were available, and each cell line (with a concentration of 0.5 × 107 cells per milliliter) was thoroughly mixed with 4% sodium hyaluronate (Hyal 2000; LG Life Sciences, Daejeon, South Korea, http://www.lgls.com) to create three different hUCB-MSCs and HA hydrogel composites. In all experiments, the hUCB-MSCs used were at passage 6. The cells expressed CD105 and CD73 but did not express CD34, CD45, CD14, or HLA-DR, in accordance with previously published data [16].
Animals
We used 6 healthy male minipigs weighing 40–45 kg and aged approximately 1.5 years. All minipigs were obtained 1 week before the experiment and raised under the same environmental conditions. All the animal experiments were approved by the Animal Care Committee of Biotoxtech Co., Ltd., at the Korean GLP facility (Ochang, Chungcheongbuk-do, South Korea). Finally, the rules put forth by the National Institutes of Health guidelines for the care and use of laboratory animals were strictly followed during the course of the present study.
Experimental Design
The experiment was performed with three available hUCB-MSC lines. Each cell line was used in 2 pigs. Anesthesia was induced by inhalation of enflurane (Geroran), combined with an intramuscular injection of xylazine (Rompun) 5 mg/kg and ketamine (Ketalar) 35 mg/kg. Both knee joint areas were shaved, cleaned with 10% betadine solution, and sterilely draped in each pig. The knee joint was opened using a medial parapatellar approach. The patella was everted laterally, and the intra-articular structures were thoroughly inspected for any abnormal findings such as infection or deformity. After confirming the normal intra-articular structure, the knee joint was fully flexed, and a full-thickness chondral injury (10 mm in diameter) was created intentionally in the trochlear groove using an arthroscopic burr. Three weeks later, the chondral injury area was reinspected. Any fibrous scar tissue filling the injured area was thoroughly removed. Using a 5-mm drill, an osteochondral defect with a depth of 10 mm was created in the middle of the chondral injury area, followed by 5 mm deep reaming of the surrounding osteochondral tissues using an 8-mm reamer. Drilling deep into the subchondral bone was confirmed by gross examination. After elimination of the cartilage, bone debris, and thrombi, a mixture (1.5 ml) of hUCB-MSCs and 4% HA hydrogel composite was transplanted into the area of the osteochondral defect in the right knee of each pig. The osteochondral defect was created in the same manner on the left knee of each pig and left untreated as the control. In order to not to cause any leakage of the transplant after the transplantation procedure, the patella retinaculum and overlying skin were carefully closed. Once a day for the next 7 days, manipulation and antibiotics (amikacin 12.5 mg/kg) were administered. Immediately afterward, an intramuscular injection of a pain reliever (ketoprofen) was given. The pigs were allowed to move the knee joints freely in a farm without any immobilization device. The clinical signs were then observed once a day during the study period. The pigs were sacrificed 12 weeks after transplantation using the identical anesthetic procedure followed by intravenous injection of potassium chloride.
Macroscopic Evaluation
After the sacrifice, each pig was placed on an operating table and shaved around the knee joint area. Arthrotomy was performed in the same manner to reinspect the intra-articular structure. After an examination for the presence of any abnormal findings suggesting rejection or infection, such as severe inflammation or extensive fibrosis, the degree of cartilage repair was grossly evaluated. The coloration, luster, irregularity, presence of any depression or bulging of the repaired tissue in the defect area, and the state of the border with the surrounding normal cartilage tissue were examined carefully.
Microscopic Evaluation
In order to histologically analyze each specimen under microscopy, hematoxylin and eosin staining, safranin O and fast green staining, and immunohistochemistry for type II collagen was performed according to the manufacturer’s instructions. Full-thickness samples (cartilage and bone) were taken from both knee joints of each pig. The specimens were fixed in 10% formaldehyde, decalcified in 10% nitric acid for 3 days, dehydrated in graded ethanol, and embedded in paraffin wax. Paraffin-embedded sections (4 µm) were cut and deparaffinized. The sections were then stained with Mayer's hematoxylin and counterstained with eosin (Dako Denmark A/S, Glostrup, Denmark, http://www.dako.com). For detection of cartilage repair, the sections were stained with a 0.1% safranin O solution (Sigma-Aldrich). For immunohistochemistry, an anti-type II collagen monoclonal antibody (EMD Millipore, Billerica, MA, http://www.emdmillipore.com) was used. The reactivity was detected using a diaminobenzidine tetrahydrochloride substrate after incubation with a horseradish peroxidase-linked secondary antibody. All samples from each staining process were mounted on coverslips with Shandon Xylene Substitute (Thermo Fisher Scientific Inc., Waltham, MA, http://www.thermofisher.com). The images of the stained section were recorded by light microscope (Nikon Eclipse 600 fitted with a digital camera [Nikon DXM1200F], Nikon, Tokyo, Japan, http://www.nikon.com).
The sections were analyzed semiquantitatively using the International Cartilage Repair Society (ICRS) grade scoring system [17]. The surface, matrix, cell distribution, cell population viability, subchondral bone, and cartilage mineralization were evaluated. Two independent researchers, unaware of any other information, completed the scoring and averaged the results. The scale consists of 6 categories, and scores are assigned from 0 to 18.
Statistical Analysis
Statistical analysis was performed using the two-tailed Mann-Whitney U test to compare the histological evaluation with Statistical Analysis Systems, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com). p Values < .05 were regarded as statistically significant.
Results
Macroscopic Findings
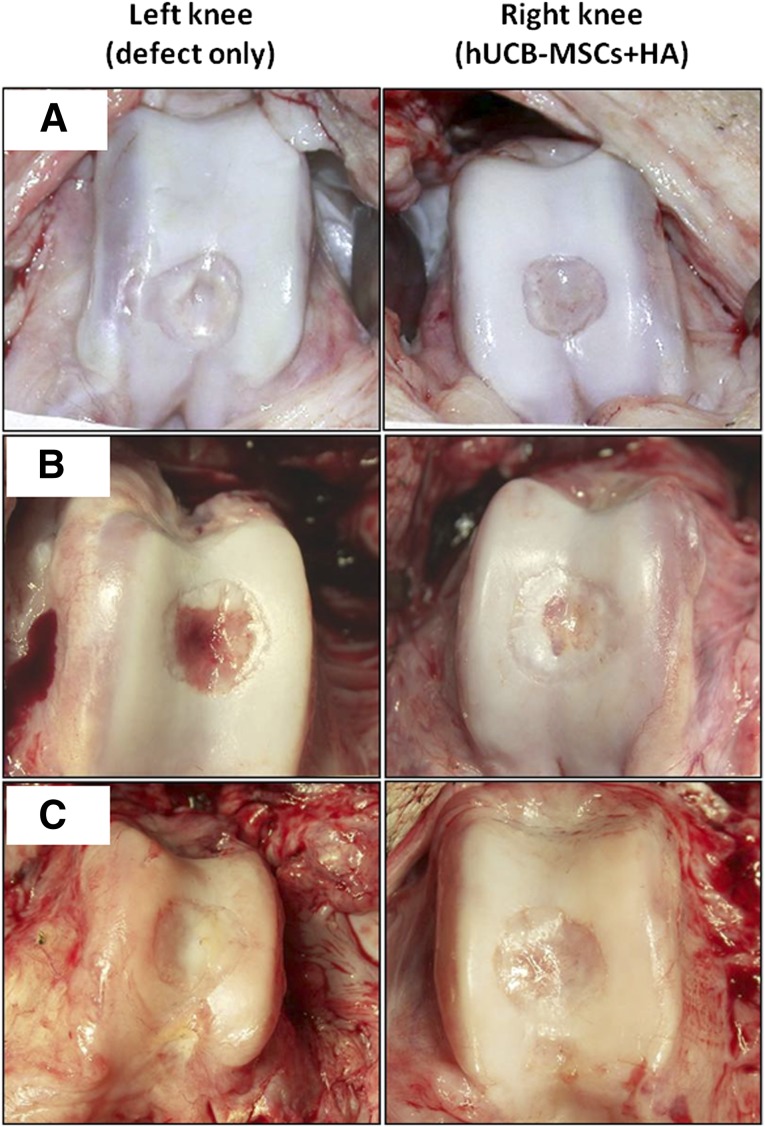
At 12 weeks after transplantation, no evidence was found of abnormal findings suggesting rejection or infection, such as severe inflammation or extensive fibrosis, in all 6 minipigs. The articular surface of the defect site in the transplanted knee was relatively smooth, with a coloration similar to that of the surrounding normal cartilage, compared with the control knee. The borderline of the defect was less distinct. Although the center of the defect was less chondrified compared with the periphery, the amount of chondrification was overall more significant than that of the left control knee. Comparing the three groups with different cell lines, no significant difference in the gross appearance was observed (Fig. 1).
Figure 1.
Macroscopic findings of the osteochondral defects of the porcine knees. At 12 weeks postoperatively, the defects of both knees had produced regenerated tissues that were pearly white and firm. These new tissues, which resembled articular cartilage, appeared adherent to the adjacent cartilage and had restored the contour of the femoral condyles (smooth articular surface without depression). The regenerated tissue of the control knee (left knee) looked fibrillated. Grossly, no difference was seen in the quality of the repaired tissue in the transplanted knee (right knee) among the three groups with different cell lines. (A): Group A. (B): Group B. (C): Group C. Abbreviations: HA, hyaluronic acid; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells.
Microscopic Findings
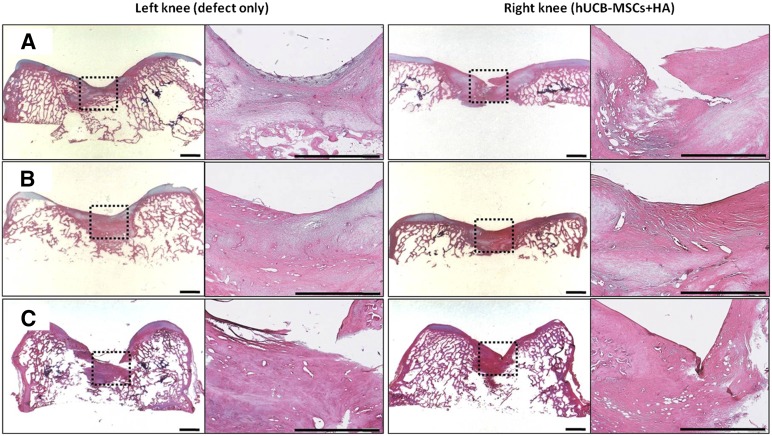
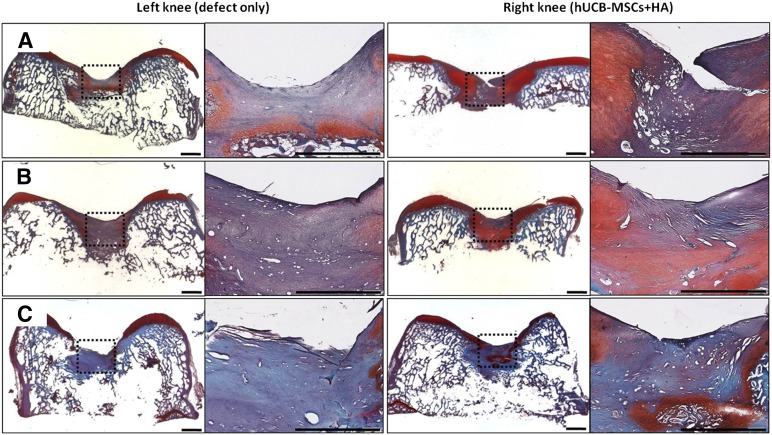
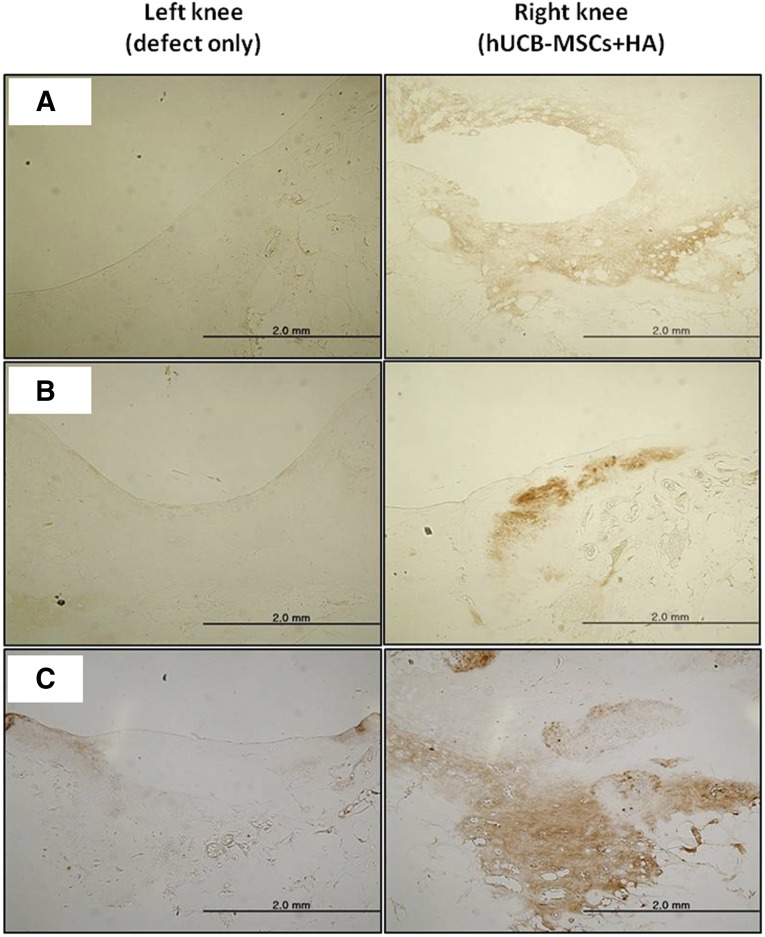
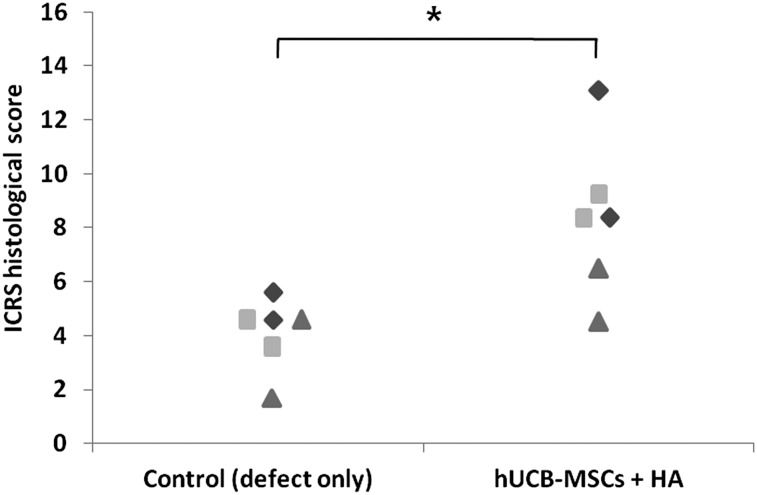
Microscopically, more cartilaginous tissues had regenerated with the surface, merging more smoothly with the surrounding normal cartilages in the transplanted knee. In the specimen stained with safranin O and fast green, the transplanted group showed more cartilaginous substances that stained denser in a broader area with the presence of lacunae. The cells in the regenerated tissues were more crowded and resembled the normal chondrocytes in the surrounding intact cartilage tissues. The differences in cell arrangement between the deep and superficial layers were more notable in the transplanted group, just as in the normal cartilage. Comparing the three groups with different cell lines, cartilage regeneration was more compatible with the characteristics described in groups A and B. Group C had a lesser amount of cartilage regeneration along the defect borderline, which was stained in a paler fashion overall. The defect of the control knee had been replaced by a relatively lesser amount of regenerated cartilage, which stained lighter with the safranin O and fast green method. The surface was irregular, with some notable gaps in parts of the regenerated tissues. The cell density was lower, with more irregular arrangement patterns compared with the transplanted group (Figs. 2, 3). In the immunohistochemical analysis for type II collagen, the control group defect area resulted in nearly pale staining, indicating the minimal production or absence of hyaline cartilage. In contrast, the transplanted group showed a more even distribution and expanded darker staining, indicating the presence of hyaline cartilage in the regenerated tissue. The differences in the degree of immunostaining among the three groups were not significant (Fig. 4). In the semiquantitative analysis of the sections using the ICRS visual histological assessment scale, the repaired tissue in the experimental knee was histologically superior overall to that in the control knee (Fig. 5).
Figure 2.
Microscopic findings of the regenerating osteochondral defects on porcine articular cartilage (hematoxylin and eosin staining). Regeneration of osteochondral defect at 12 weeks showing nearly normalized cartilage with a smooth surface and the same thickness as in the transplanted knee (right knee) but a bit of irregular surface of the cartilage in the control knee (left knee). (A): Group A. (B): Group B. (C): Group C. Scale bars = 2 mm. Abbreviations: HA, hyaluronic acid; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells.
Figure 3.
Microscopic findings of the regenerating osteochondral defects on porcine articular cartilage (safranin O and fast green staining). At 12 weeks postoperatively, the surface of the repairing tissue in the control knee (left knee) was poorly stained for glycosaminoglycan. In the transplanted knee (right knee), both the regenerated tissue and the adjacent cartilage to which it had become adherent exhibited the normal orthochromatic staining properties with safranin O. (A): Group A. (B): Group B. (C): Group C. Scale bars = 2 mm. Abbreviations: HA, hyaluronic acid; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells.
Figure 4.
Microscopic findings of the regenerating osteochondral defects on porcine articular cartilage (type II collagen immunostaining, original magnification ×40). At 12 weeks postoperatively, the regenerated tissue of the transplanted knee (right knee) had a strong affinity for the type II collagen antibody. The repaired tissue of the control knee (left knee) did not stain. (A): Group A. (B): Group B. (C): Group C. Abbreviations: HA, hyaluronic acid; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells.
Figure 5.
Histological scores of the regenerating osteochondral defects on porcine articular cartilage using the ICRS grade score at 12 weeks. The ICRS histological scores in the experimental group (hUCB-MSCs + HA) were significantly higher than those in the control group (defect only) (∗, p = .009). Diamonds indicate group A; squares, group B; and triangles, group C. Abbreviations: HA, hyaluronic acid; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; ICRS, International Cartilage Repair Society.
Discussion
In the present study, we evaluated the role of hUCB-MSCs in cartilage repair as a novel cell source. In addition, 4% HA hydrogel was evaluated for efficacy in cartilage repair, based on previous study [12, 13]. We have been investigating the cartilage regeneration potential of a hUCB-MSCs and HA hydrogel composite and attained remarkable results in rat and rabbit models. In a rat model, hUCB-MSCs with 4% HA hydrogel showed superior cartilage repair grossly and histologically compared with the control groups (HA only and defect). hUCB-MSCs with 4% HA hydrogel also showed superior cartilage repair grossly and histologically compared with the control (HA only) in a rabbit model. It seemed necessary to confirm the consistent results in a larger animal model. The present experiment was designed as an interim study to allow the procession to the human clinical trial level. At 12 weeks postoperatively, the transplantation of hUCB-MSCs and 4% HA hydrogel showed superior cartilage repair grossly and histologically. We attained favorable results for cartilage regeneration, regardless of the species. These consistent results in animal models could be a stepping stone to a human clinical trial in the future.
hUCB-MSCs can be regarded as an alternative source for cartilage regeneration. The umbilical cord contains two arteries and one vein, which are surrounded by mucoid connective tissue called Wharton’s jelly [18]. Primitive stromal cells can be isolated from the umbilical cord Wharton’s jelly and can be differentiated into different cells, including osteoblasts, chondrocytes, adipocyte, cardiomyocytes, and neurocytes [19, 20]. Compared with bone marrow and peripheral blood, hUCB contain several advantages, leading to more vigorous and frequent use as a source of mesenchymal stem cells. hUCB is easy to obtain, and the number of potential donors is high. hUCB can be collected noninvasively, avoids ethical and technical issues, and can be stored in advance and be rapidly available when needed [18]. Additionally, mesenchymal stem cells from hUCB are more primitive than MSCs isolated from some other tissue sources [21–24]. The frequency of fibroblast colony-forming units, which represents the mesenchymal progenitor cell, was also significantly higher, suggesting a greater frequency of MSCs in the umbilical cord than in bone marrow [20]. The higher proliferative capacity with a faster doubling time and consistent proliferation, even after 30 passages, might enable hUCB as an alternative source of MSCs for clinical application. Moreover, the immaturity of newborn cells, the low expression of HLA-ABC, and the absence of HLA-DR reduces graft-versus-host reactivity and might allow for the use of hUCB-MSCs in allogeneic cell therapy [23]. Considering all these characteristics, hUCB-MSCs can be regard as an alternative source for allogeneic MSCs and seem to be beneficial in off-the-shelf product transplantation. However, to date, several studies have demonstrated the chondrogenic differentiation potential of hUCB-MSCs in vitro [9, 10], and few in vivo studies are available with reasonable evidence of cartilage regeneration using hUCB-MSCs. We have previously reported that the transplantation of a composite of hUCB-MSCs and HA hydrogel resulted in favorable cartilage repair in a rat model. The results of the present study with the minipig are consistent with those of the previous study using a rat model. To our knowledge, the present study is the first in vivo study for cartilage repair with hUCB-MSCs in large animal models.
The mechanism by which MSCs and their secreted factors influence cartilage repair remains unclear. Previous studies have demonstrated MSCs have chondrogenic differentiation, and most studies using MSCs for cartilage repair have focused on MSC-mediated cartilage repair [25, 26]. In addition to the differentiation potentials, the paracrine actions of MSCs have been believed to play a role in the therapeutic effects in damaged cartilage [27]. As shown by microscopic analysis, the chondrocyte arrangement and differentiation of the transplanted group was more mature and complete in the deep zone than in the surface zone. The difference might have resulted from specific interactions between the transplanted MSCs seeded in the HA hydrogel and subchondral progenitor cells with high chondrogenic potential from the deep zone [28]. This might have been mediated via paracrine action in the presence of HA hydrogel. Recently, one study reported that hUCB-MSCs promoted differentiation of chondroprogenitor cells by paracrine action [29], suggesting the following possible therapeutic mechanisms: (a) hUCB-MSCs transplanted into cartilage lesions are stimulated by unidentified factors in synovial fluid; (b) activated hUCB-MSCs specifically increase the secretion of thrombospondin-2 as a paracrine factor; and/or (c) thrombospondin-2 promotes the differentiation of endogenous chondroprogenitor cells. Alternatively, the stimulus from MSCs in the host could be important for modulating the therapeutic activities of transplanted MSCs. Secreted proteins from mature human articular chondrocytes suppressed MSC hypertrophy during chondrogenesis [30–32]. Thus, an interaction between hUCB-MSCs and host cells plays an essential role in cartilage regeneration. To address whether the newly formed cartilage is generated by paracrine actions of the MSCs in vivo, we monitored the fate of transplanted hUCB-MSCs using immunohistochemical staining with anti-human nucleic antibody during cartilage repair in a rabbit model (ongoing submission). In that study, the transplanted hUCB-MSCs were detected at 8, but rarely at 12 weeks after transplantation. Some studies have investigated the fate of transplanted MSCs within the scaffolds of full-thickness cartilage defect or osteoarthritis in animal models [33, 34]. These studies showed that, over time after transplantation, the labeled transplanted MSCs gradually disappeared in the regenerated tissue. Although we cannot completely exclude the possibility of chondrogenic differentiation of transplanted MSCs, a certain type of interaction between hUCB-MSCs and subchondral progenitor cells initiated by paracrine action might play an essential role in cartilage regeneration.
Another underlying mechanism of cartilage repair is related to the anti-inflammatory property of hUCB-MSCs. In particular, evidence has shown that proinflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-1β, and members of the matrix metalloprotease family are induced in the superficial zone of osteoarthritis (OA) cartilage, which accelerates cartilage degradation in patients with OA [35–37]. A recent study has demonstrated that inflammation induces the expression of dickkopf-1 in OA joint tissue, leading to impaired growth and apoptosis in chondrocytes [38]. These data indicate that inflammation as an OA pathophenotype destroys the proper joint microenvironment. Singer and Caplan have demonstrated the immune modulatory function of MSCs, especially their anti-inflammation action, which might alleviate the rate of cartilage degradation in the intra-articular microenvironment. It also helps to provide a suitable condition in which chondrocytes can produce more relevant amounts of extracellular matrix (ECM) [39]. Taken together, we have assumed that a possible mechanism for cartilage regeneration in the present study is associated with chondrogenic differentiation, paracrine action, and immunomodulation of hUCB-MSCs. Although it has not been determined which of these is the main contributor.
Regarding the differences in the degree of cartilage regeneration and microscopic findings among the three groups with different cell lines, the characteristics of each cell line should be considered. During cultivation of each cell line, the cell line used in group A showed moderate cellular proliferative and an excellent chondrogenic differentiation capacity. The cell line used in group B also revealed excellent cellular proliferative and moderate chondrogenic differentiation capacity. Therefore, ideal cartilage regeneration could be expected for groups A and B. In contrast, the cell line used in group C generally resulted in poor cellular proliferative and chondrogenic differentiation capacity, resulting in inferior cartilage regeneration. According to our experimental data, transplantation of hUCB-MSCs with greater proliferative and chondrogenic differentiation capacity seemed to correlate with a greater and more complete degree of cartilage regeneration in vivo. Also, considering the comparable cartilage regeneration capacity in both groups A and B, it can be hypothesized that hUCB-MSCs with moderate chondrogenic differentiation potential could be an alternative option in vivo, as long as the cell lines express great proliferative capacity in vitro. A paracrine action or a certain unidentified interaction between the hUCB-MSCs and surrounding host cells might play a role, as described previously. A further study is anticipated.
In the present study, an HA hydrogel was used as a scaffold for transplantation of hUCB-MSCs. The main reason for using the HA hydrogel was that it could maintain the concentration of hUCB-MSCs in the defect site without dispersion. The degree of cartilage regeneration might be low for the group without an HA hydrogel, because the transplanted MSCs can scatter extensively in the intra-articular space. In addition, the transplantation strategy in which hUCB-MSCs are seeded in an HA hydrogel provides several advantages for cartilage repair owing to the biological functions and characteristics of HA. Previous studies have demonstrated improved pain relief and cartilage function after HA injection in OA patients. It appears that this was associated with certain roles of HA, such as inhibition of inflammatory factors, a reduction in cartilage degradation, the insulating effect of synovial pain fibers, and suppression of IL-1β-induced apoptosis of chondrocytes [40–42]. Furthermore, HA as an ECM might reduce the immune responses via the protection and isolation of allogeneic or xenogeneic transplanted cells from interactions with the host immune cells by producing microstructures [30]. We believe that HA itself might improve the microenvironment in defect sites, thus enhancing cartilage regeneration process by hUCB-MSC transplantation in some parts. Therefore, the HA hydrogel seems to be a good candidate as a scaffold or delivery vehicle for hUCB-MSCs to promote cartilage repair.
The limitations of our study should be addressed. First, the use of xenogeneic MSC transplantation was a nonphysiologic condition. Also, female animals were not included in the present study. Thus, we could not verify the possibility of rejection of MSC transplantation using female animals. However, the hUCB-MSCs have shown low immunogenicity and have immunomodulatory activity, suppressing the production of proinflammatory cytokines and augmenting levels of anti-inflammatory cytokines and chemokines [27, 43, 44]. Other in vivo studies using hUCB-MSCs have also shown no immune rejection [12, 13, 45]. In the present study, no evidence was found of local inflammation, joint effusion, or unloading of the joint resulting from the rejection response. Second, no control using HA only was included. HA hydrogel has been shown to support and promote the chondrogenic differentiation of MSCs [46–48]. However, some studies have shown significant differences in cartilage repair between HA only and MSC-seeded HA, and no differences between defect only and HA only in the minipig model [49, 50]. It is important to note that the presence of an exogenous cell source or chemical factors delivered from hydrogels will be needed to promote chondrogenesis and cartilage repair [51]. Because of the natural course of cartilage injury in humans, we chose to use a defect only as the control rather than HA only in the cartilage defect. Third, the mechanism of the hUCB-MSCs for regeneration of articular cartilage is currently only partially known [29]. These issues should be investigated in future studies. Fourth, a functional assessment of the knees was not performed. Quantitative magnetic resonance imaging in animals (such as T2-weighted mapping) would be expensive and technically demanding.
Conclusion
The present study has shown that the application of a composite of hUCB-MSCs with HA hydrogel to cartilage defects in a pig model results in consistent cartilage regeneration. The cellular characteristics of hUCB-MSCs influenced the degree of cartilage regeneration potential. Although additional studies are needed to elucidate the precise underlying mechanisms, including chondrogenic differentiation, paracrine action, and immunomodulation in detail, these findings suggest that hUCB-MSCs with HA hydrogel can be used for the regenerative treatment of full-thickness articular cartilage defects. This evidence of consistent cartilage regeneration in a large animal model could be a stepping stone to a human clinical trial in the future.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI14C3484). The funding sources had no involvement in the study design, data collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
Author Contributions
C.-W.H.: conception/design, manuscript writing, data analysis and interpretation, final approval of manuscript; Y.-B.P.: conception/design, manuscript writing, provision of study material or patients, data analysis and interpretation; J.-Y.C.: manuscript writing, collection and/or assembly of data; Y.-G.P.: provision of study material or patients, data analysis and interpretation.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Mobasheri A, Csaki C, Clutterbuck AL, et al. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: Applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–366. doi: 10.14670/HH-24.347. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 3.Wolf D, Wolf AM. Mesenchymal stem cells as cellular immunosuppressants. Lancet. 2008;371:1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 5.Ende N, Lu S, Mack R, et al. The feasibility of using blood bank-stored (4 degrees C) cord blood, unmatched for HLA for marrow transplantation. Am J Clin Pathol. 1999;111:773–781. doi: 10.1093/ajcp/111.6.773. [DOI] [PubMed] [Google Scholar]
- 6.Arien-Zakay H, Lazarovici P, Nagler A. Tissue regeneration potential in human umbilical cord blood. Best Pract Res Clin Haematol. 2010;23:291–303. doi: 10.1016/j.beha.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Mara CS, Duarte AS, Sartori A, et al. Regulation of chondrogenesis by transforming growth factor-beta 3 and insulin-like growth factor-1 from human mesenchymal umbilical cord blood cells. J Rheumatol. 2010;37:1519–1526. doi: 10.3899/jrheum.091169. [DOI] [PubMed] [Google Scholar]
- 9.Kao I-T, Yao C-L, Chang Y-J, et al. Chondrogenic differentiation of human mesenchymal stem cells from umbilical cord blood in chemically synthesized thermoreversible polymer. Chin J Physiol. 2008;51:252–258. [PubMed] [Google Scholar]
- 10.Choi YS, Im MW, Kim CS, et al. Chondrogenic differentiation of human umbilical cord blood-derived multilineage progenitor cells in atelocollagen. Cytotherapy. 2008;10:165–173. doi: 10.1080/14653240701817002. [DOI] [PubMed] [Google Scholar]
- 11.Hildner F, Wolbank S, Redl H, et al. How chondrogenic are human umbilical cord matrix cells? A comparison to adipose-derived stem cells. J Tissue Eng Regen Med. 2010;4:242–245. doi: 10.1002/term.236. [DOI] [PubMed] [Google Scholar]
- 12.Chung JY, Song M, Ha CW, et al. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5:39. doi: 10.1186/scrt427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YB, Song M, Lee CH, et al. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. doi: 10.1002/jor.22950. 2015. [DOI] [PubMed] [Google Scholar]
- 14.Humphray SJ, Scott CE, Clark R, et al. A high utility integrated map of the pig genome. Genome Biol. 2007;8:R139. doi: 10.1186/gb-2007-8-7-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groenen MA, Archibald AL, Uenishi H, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393–398. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S-E, Ha C-W, Jung M, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 17.Mainil-Varlet P, Aigner T, Brittberg M, et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A(suppl 2):45–57. [PubMed] [Google Scholar]
- 18.Malgieri A, Kantzari E, Patrizi MP, et al. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L-F, Wang N-N, Liu Y-S, et al. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2009;15:2865–2873. doi: 10.1089/ten.TEA.2008.0579. [DOI] [PubMed] [Google Scholar]
- 20.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 21.Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–616. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 22.Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 23.Lu L-L, Liu Y-j, Yang S-G, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 24.Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto T, Muneta T, Tabuchi T, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Tang J, Wang R, et al. Comparing the chondrogenic potential in vivo of autogeneic mesenchymal stem cells derived from different tissues. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:31–38. doi: 10.3109/10731191003776769. [DOI] [PubMed] [Google Scholar]
- 27.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 28.Neumann K, Dehne T, Endres M, et al. Chondrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral cortico-spongious bone. J Orthop Res. 2008;26:1449–1456. doi: 10.1002/jor.20635. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SY, Kim DH, Ha J, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–2148. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 30.Steck E, Fischer J, Lorenz H, et al. Mesenchymal stem cell differentiation in an experimental cartilage defect: Restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 31.Giovannini S, Diaz-Romero J, Aigner T, et al. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater. 2010;20:245–259. doi: 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]
- 32.Fischer J, Dickhut A, Rickert M, et al. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 33.Koga H, Muneta T, Ju YJ, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J, Schmitt-Talbot E, DiMattia DA, et al. The differential effects of IL-1 and TNF-α on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm Res. 2004;53:377–389. doi: 10.1007/s00011-004-1271-3. [DOI] [PubMed] [Google Scholar]
- 36.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes JC, Martel-Pelletier J, Pelletier J-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 38.Weng L-H, Wang C-J, Ko J-Y, et al. Inflammation induction of Dickkopf-1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthritis Cartilage. 2009;17:933–943. doi: 10.1016/j.joca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 40.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]
- 41.Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62:565–572. [PubMed] [Google Scholar]
- 42.Curran MP. Hyaluronic acid (Supartz®): A review of its use in osteoarthritis of the knee. Drugs Aging. 2010;27:925–941. doi: 10.2165/11205920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Flynn A, Barry F, O’Brien T. UC blood-derived mesenchymal stromal cells: An overview. Cytotherapy. 2007;9:717–726. doi: 10.1080/14653240701584578. [DOI] [PubMed] [Google Scholar]
- 44.Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: Cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 45.Lee M, Jeong SY, Ha J, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983–989. doi: 10.1016/j.bbrc.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astachov L, Vago R, Aviv M, et al. Hyaluronan and mesenchymal stem cells: From germ layer to cartilage and bone. Front Biosci (Landmark Ed) 2011;16:261–276. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 48.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials. 2011;32:8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher MB, Belkin NS, Milby AH, et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: Implications for tissue engineering. Tissue Eng Part A. 2015;21:850–860. doi: 10.1089/ten.tea.2014.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee KB, Hui JH, Song IC, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—A porcine model. Stem Cells. 2007;25:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 51.Bian L, Zhai DY, Tous E, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]