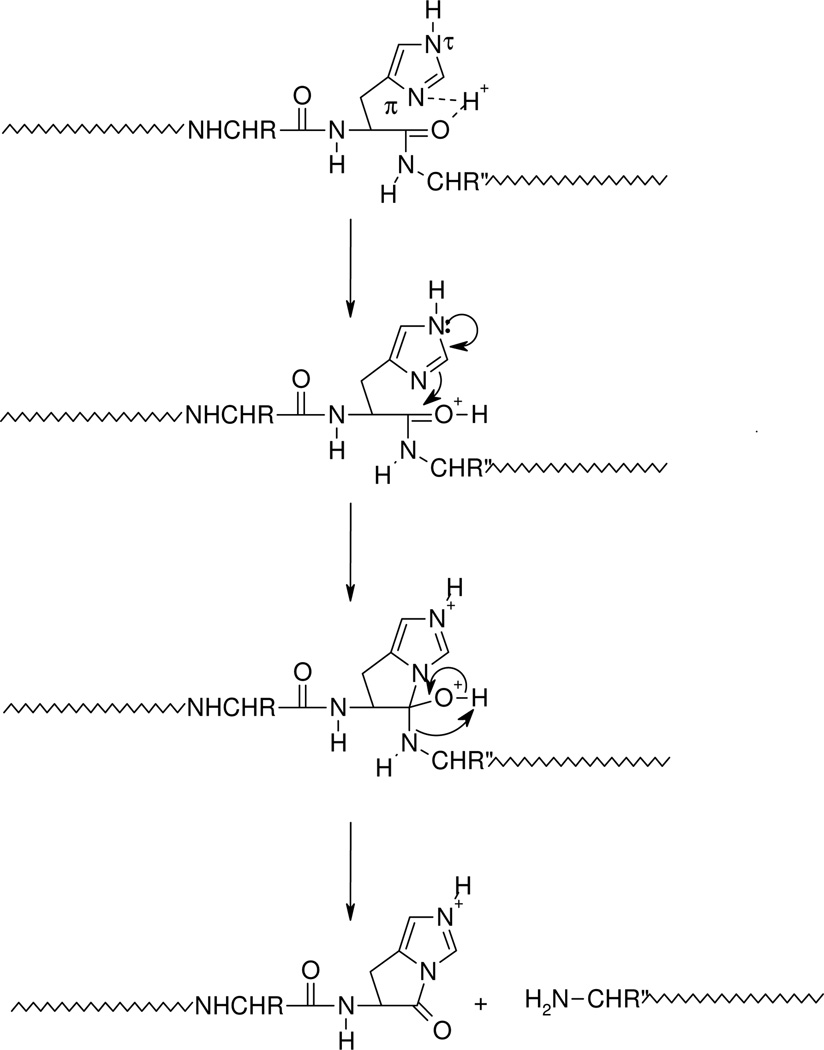
Scheme 1.
Proposed mechanism for the formation of the histidine b ion. Transfer of the ionizing proton by protonated histidine to the peptide oxygen renders the carbonyl carbon electropositive. Nucleophilic attack by the imino nitrogen of histidine on the electropositive carbonyl carbon along with N-protonation leads to breaking of the peptide bond and the fused bicyclic ring b ion structure. The C-terminal leaving piece is neutral.