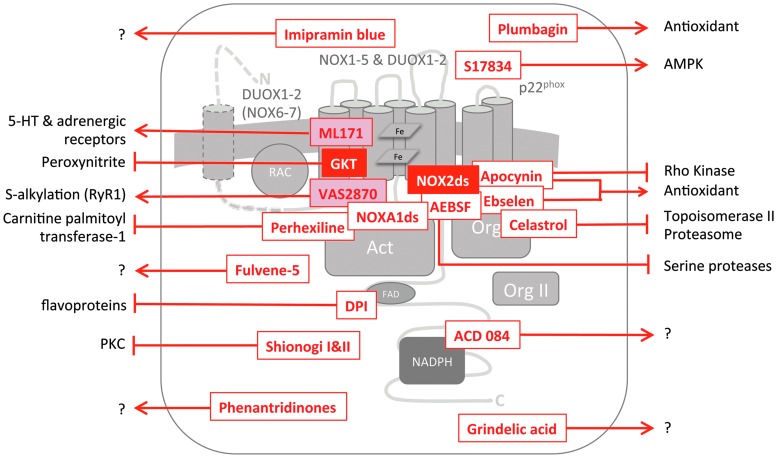
FIG. 2.
Mechanisms of NOX inhibition. The scheme shows the general structure of NAPDH oxidase complexes with the catalytic subunits, NOX1-7, in the plasma membrane, the membrane-bound binding partner, p22phox, one or two organizer binding proteins (Org I and Org II), the small GTPase, Rac, and one activator binding protein (Act). The cytosolic NOX C-termini have an FAD and an NAPDH binding domain. DUOX1/NOX6 and DUOX2/NOX7 have an additional transmembrane domain and an extracellular N-terminus. Suggested, but not completely validated or unspecific inhibitors of NAPDH oxidase activity are shown in red font, recommended inhibitors in white font on a red background, and partly recommended inhibitors in red font on a pale red background. Inhibitors are recommended if they are specific for NADPH oxidases and show efficacy in cell-free, cellular, and in vivo conditions. Placement of the inhibitors indicates their likely point of interaction, arrows indicate off-target effects, and arrows with question marks indicate insufficient characterization regarding off-target effects. The NOX2ds-tat peptide (145) and AEBSF (42) prevent complex assembly of the respective NOX isoform with its organizer subunit, in case of NOX2ds-tat, NOX2, and p47phox. AEBSF also inhibits serine proteases. Celastrol (76), ebselen (164), and apocynin (123, 166) inhibit the binding of the organizer proteins NOXO1 and p47phox to p22phox. Ebselen and apocynin are known ROS scavengers. The latter also inhibits rho kinases. Celastrol further inhibits topoisomerase II and the proteasome. NOXA1ds inhibits binding of the respective NOX activator, NOXA1 to NOX1. VAS2870 is very likely an assembly inhibitor with a yet unknown target domain (3). It was shown to alkylate cysteine residues in the RyR1 receptor (167). DPI is a flavoprotein inhibitor. Since ACD 084 inhibited ROS from the NOX4 dehydrogenase domain (89), it may act either on the FAD or NAPDH binding site or as a direct antioxidant. The Shionogi compounds are not NOX inhibitors but prevent the assembly of NADPH oxidase complexes indirectly by the inhibition of protein kinase C (55). Imipramin is a cation and can, therefore, not cross cell membranes. It most likely exerts its NOX inhibition extracellularly. S17834 and plumbagin are polyphenols and most likely scavenge ROS directly. The main target of S17834, however, seems to be AMPK. No mechanisms of action are published for the GKT compounds (GKT136901 and GKT137831) and ML171. GKT136901 scavenges peroxynitrite, and ML171 was reported to inhibit serotonin and adrenergic receptors with very low affinity and potency. AEBSF, 4-(2-aminoethyl)- benzenesulphonyl fluoride; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; DPI, diphenylene iodonium; FAD, flavin adenine dinucleotide; NOX2ds, NOX2 docking sequence; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars