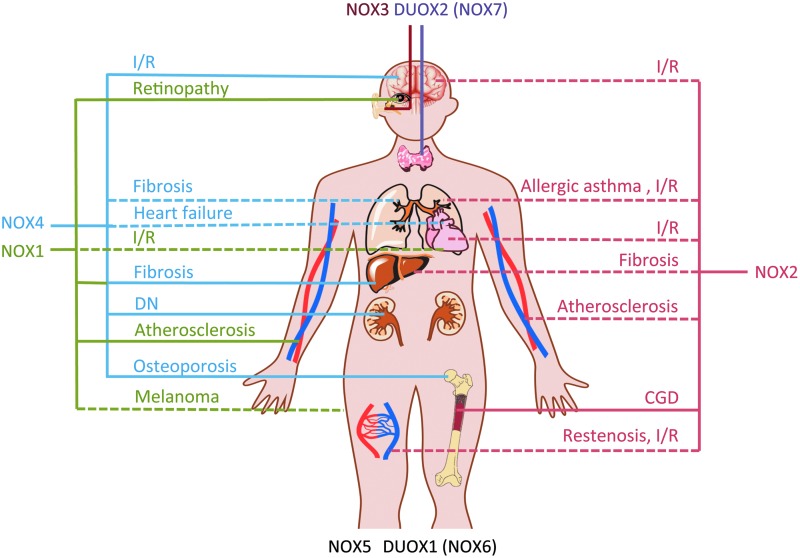
FIG. 7.
NOX enzymes as validated therapeutic targets. The validation status of NOX1–7 is presented based on partial validation of the mentioned disease model in knockout animals or by using NOX inhibitors (dashed lines), or—for full validation—based on both knockout animals and NOX inhibition or knowledge on mutations leading to human disease (full lines). Knockout mice and inhibition studies strongly suggested NOX1 (green lines) as a therapeutic target in diabetic atherosclerosis (64), ischemic retinopathy (179), and—in interaction with NOX4—liver fibrosis (5, 79, 129). A role in melanoma progression and tumor angiogenesis (54) needs to be confirmed in NOX1 knockout mice, while a role of NOX1 in heart I/R injury (21) needs to be confirmed by pharmacologic inhibition of NOX1. NOX2 (red lines) is suggested to be involved in almost every animal disease model, especially involving inflammatory components. The involvement in CGD is based on human disease and, therefore, validated. A likely role of NOX2 in atherosclerosis, endothelial dysfunction, and restenosis after arterial injury is based on both NOX2 knockout animals (30, 81) and studies with the NOX2ds-tat peptide (46, 74, 177, 194). NOX2 should, therefore, be further considered a target for atherosclerosis and restenosis after arterial injury. A minor role for NOX2 was found in I/R injury of several organs, including the brain [reviewed in ref. (140)], lung (178), and heart (51, 101), but this is currently insufficiently validated. The role of NOX2 in liver fibrosis (129) and allergic asthma (6) is awaiting confirmation with specific NOX2 inhibitors. NOX3's (brown line) physiological role in the inner ear can be considered fully validated in the mouse model, as NOX3 specific inhibition prevented cisplatin-induced hearing loss. NOX4 (blue lines) is a valid therapeutic target for stroke (88), diabetic nephropathy (78), liver fibrosis (5, 79), and osteoporosis (60), at least in mice. The validation of NOX4's role in lung fibrosis and heart failure needs to be confirmed using specific NOX inhibitors and knockout animals in a relevant model. For NOX5, a role in spermatozoa motility was suggested based on the inhibition with GKT136901 (118). DUOX1/NOX6 knockout animals do not show an obvious phenotype, but roles in the bladder (45) or in the lung host defence system were suggested (173). Dysfunctional DUOX2/NOX7 (purple line) due to bi-allelic mutations in humans leads to severe hypothyroidism. CGD, chronic granulomatous disease; I/R, ischemia-reperfusion. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars