Figure 6.
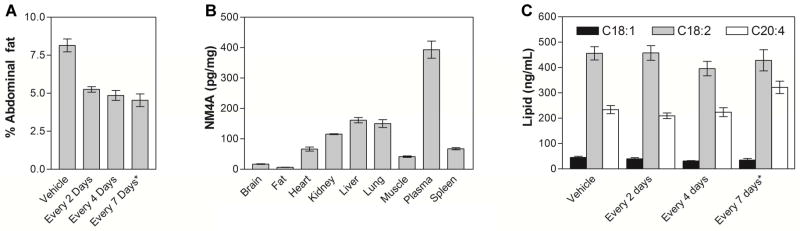
(A) Abdominal fat of chronically treated animals. Ad libitum fed male DIO C57BL/6 mice were treated with either vehicle or NM4A (2.2 mg/kg; 1603 nmoles/kg) every 2 days or NM4A (2.2 mg/kg; 1603 nmoles/kg) every 4 days or (*) NM4-C16 (2.6 mg/kg; 1603 nmoles/kg) every 7 days for 21 days. Subsequently, their abdominal fat was harvested and weighed. Results are expressed as abdominal fat as a % of total body weight. (B) Distribution of NM4A in NM4A chronically treated animals (2.2 mg/kg; every 2 days for 28 days). Subsequently their organs were harvested and analyzed using MDS Sciex QSTAR XL Hybrid Quadrupole Time-of-Flight LC/MS (Applied Biosystems) with methylated NM4A (NM4A-Me) as an internal standard. (C) LPA levels in the plasma of chronically treated animals with either vehicle or NM4A (2.2 mg/kg; 1603 nmoles/kg) every 2 days or NM4A (2.2 mg/kg; 1603 nmoles/kg) every 4 days or (*)NM4-C16 (2.6 mg/kg; 1603 nmoles/kg) every 7 days for 21 days. (18:1)-1-oleoyl-2-hydroxy-sn-glycero-3-phosphatidic acid, (18:2)-1-linoleoyl-2-hydroxy-sn-glycero-3-phosphatidic acid, (20:4)-1-arachidonoyl-2-hydroxy-sn-glycero-3-phosphatidic acid.
