Abstract
Evaluating whether a new medication prolongs QT intervals is a critical safety activity that is conducted in a sensitive animal model during non-clinical drug development. The importance of QT liability detection has been reinforced by non-clinical [International Conference on Harmonization (ICH) S7B] and clinical (ICH E14) regulatory guidance from the International Conference on Harmonization. A key challenge for the cardiovascular safety community is to understand how the finding from a non-clinical in vivo QT assay in animals predicts the outcomes of a clinical QT evaluation in humans. The Health and Environmental Sciences Institute Pro-Arrhythmia Working Group performed a literature search (1960–2011) to identify both human and non-rodent animal studies that assessed QT signal concordance between species and identified drugs that prolonged or did not prolong the QT interval. The main finding was the excellent agreement between QT results in humans and non-rodent animals. Ninety-one percent (21 of 23) of drugs that prolonged the QT interval in humans also did so in animals, and 88% (15 of 17) of drugs that did not prolong the QT interval in humans had no effect on animals. This suggests that QT interval data derived from relevant non-rodent models has a 90% chance of predicting QT findings in humans. Disagreement can occur, but in the limited cases of QT discordance we identified, there appeared to be plausible explanations for the underlying disconnect between the human and non-rodent animal QT outcomes.
Table of Links
| TARGETS |
|---|
| Ion Channels |
| Kv11.1 (KCNH2) |
| LIGANDS |
|---|
| Diphenhydramine |
| Famotidine |
| Nifedipine |
| Verapamil |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
The potential of a new drug to prolong the QT interval is a major safety concern evaluated extensively during non-clinical and clinical drug development because it is a significant risk factor for torsade de pointes (TdP), a polymorphic ventricular arrhythmia that can be either self-limiting or, in some cases, lethal (Shah, 2002; Darpo, 2010; Pollard et al., 2010). Drug-induced prolonged QT intervals in humans are usually caused by the drug's ability to inhibit IKr, the rapid component of the delayed rectifier potassium current. In humans, this component is encoded by human ether-a-go-go related gene (hERG), also known as Kv11.1 (KCNH2; Redfern et al., 2003). New drugs are evaluated for IKr inhibitory potency using voltage clamp studies of hERG function in vitro, and their ability to prolong QT intervals in humans is evaluated in a non-rodent animal model (Redfern et al., 2003; Hanson et al., 2006; Pollard et al., 2010) in accordance with non-clinical [International Conference on Harmonization (ICH) S7B] and clinical (ICH E14) guidelines for drug-induced pro-arrhythmia (Anonymous, 2005a,b,). These regulatory guidelines have changed the way cardiovascular safety evaluations of drug candidates are conducted and are responsible for the recent successful development of new drugs with low pro-arrhythmic risk (Park et al., 2013).
Although we understand how hERG channel inhibition can prolong ventricular action potential duration and the QT interval in vivo, most of this knowledge has been derived from retrospective studies of human torsadogenic drugs in animal models (Omata et al., 2005; Hanson et al., 2006). Another approach is to assess the relationship between non-clinical and clinical QT datasets for the new drugs submitted to the Food and Drug Administration (FDA) since the new ICH guidelines were implemented. The Health and Environmental Sciences Institute (HESI) Cardiovascular Pro-Arrhythmia Working Group (Pro-AWG) supported the creation of a FDA database to determine the concordance between non-clinical QT risk models and the human TQT (thorough QT/QTc study described by ICH E14) study (Trepakova et al., 2009; Pierson et al., 2013).
The Pro-AWG approach to concordance has many advantages including (i) review of a large number of pharmacologically diverse drugs, and (ii) the use of contemporary non-clinical methods to gather data, such as telemetry in conscious dogs or non-human primates (NHPs). However, a potential limitation of the FDA-TQT dataset is that it might be weighted heavily with low QT prolongation risk drugs, since drugs with a high risk for QT interval prolongation are typically terminated early in drug development (Pollard et al., 2010). This bias was substantiated by a recent TQT trend analysis that found that 78% of recent new drug submissions to the FDA (n = 205) had no effect on the QT interval, and most of the positive drugs exhibited only small to moderate increases in QT intervals (Park et al., 2013).
In this study, we conducted a literature search of human and animal studies on drugs with non-clinical and clinical QT interval data to assess the concordance between clinical and non-clinical study findings in a reduced-biased dataset. Past literature-based assessments of this problem have shown that non-rodent animal models accurately demonstrate QT interval prolongation and TdP caused by human therapeutics (Davis, 1998; De Ponti et al., 2001; Webster et al., 2002; Redfern et al., 2003). Because these prior evaluations focused on drugs with profound QT liability and torsadogenic potential (i.e. terfenadine and cisapride) that were withdrawn from human use, we looked for studies that augmented the FDA-TQT database by using drugs that may have been dropped from clinical development because of human QT prolongation risk or drugs that were developed prior to the emergence of ICH S7B and E14 guidance documents. For example, the novel dual dopaminergic-adrenergic receptor agonist Viozan™ (sibenadet) was a new chemical entity terminated in phase III clinical trials. Although non-clinical studies demonstrated no QT interval prolongation and low proarrhythmic risk, both of these phenomena occurred during human testing (Valentin et al., 2006; Newbold et al., 2007). A preliminary account of this literature review was presented in abstract form (Vargas et al., 2012).
Literature search based on text mining
The literature search was conducted to identify drugs that prolong the QT interval in both humans and animals known to respond to hERG inhibition (rabbits, guinea pigs, dogs and NHPs). Other animal species (minipigs, ferrets) were not included because of their infrequent use for QT risk assessment.
Typical literature searches rely upon specific keywords to identify publications of interest, but we used a semantics-based text-mining approach to identify relevant drugs (Spasic et al., 2005). The Safety Intelligence Program (SIP, www.instem.com) was used to identify the relationships between drugs of interest and observations of QT prolongation. The SIP is an industry-sponsored initiative designed to produce assertional metadata that captures detailed relationships between drugs and biomedical findings mined from public sources. The SIP has been used to evaluate hepatic adverse events (Fourches et al., 2010), and can evaluate other organ system findings, such as cardiovascular toxicity. The SIP uses an extensive lexicon of terms and synonyms extracted from the published literature, and is augmented with synonyms from additional sources such as ChEMBL, PubChem and Medline. The program mines text by using ‘sentence-like’ search statements (noun-verb-subject) that are represented in triple constructs (concept-relationship-concept). The verbal relationships between concepts are retrieved from the SIP knowledge base as assertions. In our study, the assertions describe the effect of a particular drug on the QT interval based on parts of speech (e.g. ‘Cisapride_is related to QT prolongation.’ The Sophia™ interface of SIP allows the user to filter the metadata by drug, literature source, species, or a variety of other criteria. We used the text-mining approach to evaluate public literature from published digital sources [PubMed, FDA reports, the Adverse Event Reporting System (AERS), etc.] between 1960 and 2011.
The central question of the literature search was ‘What is the concordance of drug-induced QT prolongation in selected non-clinical and clinical studies?’ To answer this question, a list of key words or short phrases associated with QT prolongation was compiled by searching the SIP database for matches with QT-related terms. This was followed by curation by four expert reviewers who added missing terms and subtracted non-specific terms. The final list of 180 terms or phrases (including synonyms) was then used to search the SIP database to identify a list of drugs and their relationship to the QT interval concepts in the term list. The Pro-AWG literature search followed the approach and process outlined in Figure 1.
Figure 1.
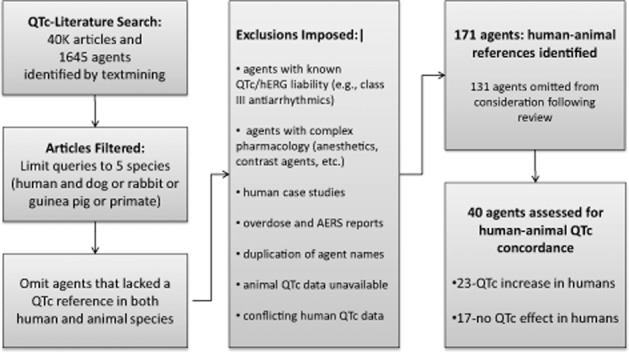
An overview of the QTc literature search: process flow chart.
The initial literature search yielded over 40 000 assertions on approximately 1600 drugs and combinations of drug products. The results were refined by eliminating assertions from AERS data, which is not as rigorously evaluated as data in peer-reviewed literature, and was also filtered to focus on studies that used monkeys, dogs, rabbits and guinea pigs, all of which have demonstrated sensitivity to hERG blockade QT prolongation (Redfern et al., 2003; Ando et al., 2005; Toyoshima et al., 2005; Hanson et al., 2006; Guth, 2007). The process yielded 591 drugs of interest that were reviewed further and curated based on predefined inclusion and exclusion criteria.
Inclusion criteria
The minimal requirement for inclusion in this literature survey was that each drug had been evaluated for its effect on the QT interval in published investigations in both humans and animal species with known sensitivity to hERG channel blockade. The QT signal could be positive (prolonged) or negative (not prolonged). The published non-clinical QT data were included irrespective of the animal model conditions (anaesthesia, restrained or unrestrained, conscious), the drug treatment protocol, the ECG methodology used to monitor the QT interval or the heart rate correction formula. The human QT data were included irrespective of study design, study duration, sample size, specific clinical subject population enrolled, QT data collection method, QT analysis extraction method or QT correction method used because the clinical QT evaluations were performed both before and after the implementation of ICH E14 requirements. The significance of the human and animal QT findings was accepted as reported.
Exclusion criteria
In this analysis, drugs were removed from consideration for several reasons, including
QT data were reported for only one species (either human or animal).
The drugs were developed as class III anti-arrhythmic drugs designed to increase QT interval duration (dofetilide, ibutilide, etc.). These drugs were excluded to avoid a positive bias in the concordance analysis.
Studies that evaluated the primary effect of various anaesthetics (isoflurane, pentobarbital, etc.) on ECG or QT intervals because these agents have complex effects on cardiac and neuronal electrophysiology.
Human QT evaluations conducted as drug–drug interaction studies because the pharmacokinetic and pharmacodynamic factors in such studies impact study interpretation significantly.
Human case reports of QT or TdP (or AERS) because these studies are not controlled, and may involve multiple drug combinations that lack a non-clinical correlate.
Diagnostic pharmaceuticals and/or imaging-contrast agents (meglumine sodium diatrizoate, iohexol, etc.) because they lack a non-clinical correlate.
Reports that referred to QT shortening.
Protein-based therapeutics because these agents rarely prolong the QT interval (Vargas et al., 2008).
Definition of concordance
For the purposes of this analysis, concordance was defined as the demonstration of QT prolongation, or the absence of such an effect, in both the human and animal (one or more species) study (Valentin et al., 2009). Discordance was defined as opposite findings in humans and the animal species used in the study. The sensitivity, specificity and overall accuracy (predictability) of the non-clinical assays were calculated in percentages as (Gintant, 2011):
Sensitivity = true positive (TP)/[TP + false negative (FN)]
Specificity = true negative (TN)/[TN + false positive (FP)]
Overall accuracy = (TP + TN)/(TP + TN + FP + FN)
Results
The clinical-non-clinical QT dataset
To calculate QT concordance, we used filters to extract and refine human and animal QT data from the literature. Applying the exclusion criteria left 171 drugs to evaluate for QT findings in the species of interest (Figure 1). A more thorough review of the published reports eliminated 131 of these drugs (Figure 1) because they had uninterpretable human QT findings (dosulpin, ethmozine, falipamil, etc.), they were clinical case studies instead of controlled clinical trials (doxepin, methoxamine, etc.) or they had inadequate animal QT data assessments. There were 40 drugs in the final dataset, 23 of which prolonged the QT interval in humans and 17 that did not.
It should be noted that, in an effort to expand the literature-based data, all 40 drugs were profiled for their pharmacological activity in hERG function and trafficking assays (see Supporting Information Table S1).
Concordance of clinical and non-clinical QT findings: drugs that cause QT interval prolongation
The 23 drugs reported to prolong the QT interval in humans came from a broad range of therapeutic classes (Table 2013). Some of the drugs are in current clinical use, while others were evaluated only in limited clinical trials or are no longer on the market. Ninety-one percent (21/23) of these drugs prolonged the QT interval in animal models as well (Table 2013), which indicates that the animal models have high sensitivity. Based on the year of publication, many of the studies included in this search were conducted prior to 2005, the year when ICH S7B and E14 were implemented (Anonymous, 2005a,b,).
Table 1.
Literature search agents (23) reported to prolong the QTc interval in humans: concordance with animal QTc studies
| Agent | Clinical use | ↑QTc human | ↑QTc animal | Referencesa |
|---|---|---|---|---|
| Amantadine | Antiviral | + | + | (h): L Wu Poisoning & Drug Overdose (KR Olson, Ed.) (2007) |
| Parkinson's disease | (g): M Hiraoka et al. Circ Res 65:880–893 (1989). | |||
| Arsenic trioxide | Anti-cancer | + | + | (h): J Zhou et al., Chin Med J (Engl) 116:1764–6 (2003) |
| (g): HL Sun et al., Basic Clin Pharmacol Toxicol 98:381–8 (2006) | ||||
| Astemizole | Antihistamine | + | + | (h): YG Yap et al. Clin Allergy Immunol 17:389–419 (2002) |
| (d): JJ Salata et al., Circ Res 76:110–19 (1995) | ||||
| Atomoxetine | Antidepressant | + | + | (h): STRATTERA™ (atomoxetine) FDA Review & Evaluation of Clinical Data (NDA 21–411), 2002 |
| (d): STRATTERA™ (atomoxetine) FDA Pharmacology Review (NDA 21–411), 2002 | ||||
| Bepridil | Anti-anginal | + | + | (h): B Lecocq et al., Am J Cardiol 66:636–41.(1990) |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Cisapride | Antihistamine | + | + | (h): AD van Haarst et al. Clin Pharmacol Ther 64:542–6 (1998) |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Citric acid (citrate) | Excipient | + | + | (h): SJ Laspina et al., Transfusion 42:899–903 (2002) |
| (d): T Fukuda et al., Clinical Nutrition 25:984–993 (2006) | ||||
| Clobutinol | Anti-tussive | + | + | (h): C Belloca et al., Mol Pharmacol 66:1093–1102 (2004). |
| (g): A Takahara et al., J Cardiovasc Pharmacol 54:552–559 (2009) | ||||
| Dasatinib | Anti-cancer | + | + | (h): GS Orphanos et al., Acta Oncol 48:964–970 (2007). |
| (r): SPRYCEL™ (dasatinib) FDA Pharmacology/Toxicology Review & Evaluation (NDA 21–986 & 22–072), 2005 | ||||
| Diphenhydramine | Antihistamine | + | − | (h): W Zareba et al., Am J Cardiol 80:1168–73 (1997). |
| (g): JA Hey et al., Clin Exp Allergy 25:974–84 (1995). | ||||
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–71 (2005); LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Doxorubicin | Anti-cancer | + | + | (h): T Nousianen et al., J Intern Med 245:359–64 (1999). |
| (r): P Milberg et al., Basic Res Cardiol 102:42–51 (2007) | ||||
| DPI 201-106 | Inotrope | + | + | (h): V Kühlkamp V et al., J Cardiovasc Pharmacol 42:113–117 (2003). |
| (d): MJ Walker et al., J Cardiovasc Pharmacol 14:381–388 (1989). | ||||
| Famotidine | Antihistamine | + | − | (h): KW Lee et al., Am J Cardiol 93:1325–7 (2004). |
| (d): A Sugiyama et al., Eur J Pharmacol 466:137–46 (2003) | ||||
| Haloperidol | Antipsychotic | + | + | (h): PJ Weiden et al., J Clin Psychopharmacol 28:S12–9 (2008) |
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–71 (2005) | ||||
| Melperone | Antipsychotic | + | + | (h): WK Hui et al., J Cardiovasc Pharmacol 15:144–9 (1990); C Stollberger et al., Int Clin Psychopharmacol 20(5):243–51 (2005). |
| (d) ES Platou et al., Can J Physiol Pharmacol 64:1286–90 (1986) | ||||
| Papaverine | Anti-anginal | + | + | (h): MJ Kern et al., Cathet Cardiovasc Diagn 19:229–36 (1990). |
| (d): CW Christensen et al., Circulation 83:294–303 (1991) | ||||
| Pentamidine | Anti-protozoal | + | + | (h): MD Eisenhauer et al., Chest 105:389–395 (1994). |
| (d): H Yokoyama et al., J Pharmacol Sci 110:476–82 (2009) | ||||
| Pimozide | Antipsychotic | + | + | (h): G Fulop et al., Am J Psychiatry 144:673–675 (1987) |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Probucol | Anti-hyperlipidemia | + | + | (h): KF Browne et al., Am Heart J 107:680–4 (1984) |
| (m): JE Lebeau. Nouv Presse Med 9:3001–4 (1980) | ||||
| Sildenafil | Erectile dysfunction | + | + | (h): J Morganroth et al., Am J Cardiol 93:1378–83 (2004) |
| (d): O Nagy et al., Br J Pharmacol 141:549–51 (2004) | ||||
| Tacrolimus | Immuno-suppression | + | + | (h): SP Hodak et al., Transplantation 66:535–7 (1998); MC Johnson et al., Transplantation 53:929–30 (1992) |
| (g): T Minematsu et al., Pharmacokinet Pharmacodyn 28:533–54 (2001) | ||||
| Terfenadine | Antihistamine | + | + | (h): BP Monahan et al., JAMA. 264:2788–2790 (1990) |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Thioridazine | Antipsychotic | + | + | (h): AH Glassman & JT Bigger Jr. Am J Psychiatry 158:1774–82 (2001) |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) |
The reference corresponds to a representative QTc study conducted in humans (h), primates (m), dogs (d), rabbits (r), guinea pigs (g).
The analysis showed that diphenhydramine and famotidine demonstrated discordance between the reported clinical and non-clinical QT data, that is, a QT-positive signal was identified in a human QT study, but not in an animal cardiovascular safety model (dogs and guinea pigs for diphenhydramine and dogs for famotidine). A general observation was dogs were used frequently in non-clinical QT evaluation studies.
Concordance of clinical and non-clinical QT findings: drugs that have no effect on the QT interval
The literature review identified 17 drugs that did not prolong the QT interval in humans (Table 2005). Fifteen of these drugs (88%) also did not prolong the QT interval in an animal model (Table 2005), which indicates that the animal models have high specificity. Nifedipine and verapamil did not prolong the QT interval in humans but did prolong it in a conscious telemetered dog model.
Table 2.
Literature search agents (17) reported to have no effect on the QTc interval in humans: concordance with animal QTc studies
| Agent | Clinical use | ↑QTc human | ↑QTc animal | Referencesa |
|---|---|---|---|---|
| Alinidine | Negative chronotrope | − | − | (h): UW Wiegand et al., J Cardiovasc Pharmacol 4:59–62 (1982) |
| (d): Traunecker W, Walland A, Arch Int Pharmacodyn Ther 244:58–72 (1980) | ||||
| Almotriptan | Anti-migraine | − | − | (h): M Boyce et al., J Cardiovasc Pharmacol 37:280–289 (2001) |
| (d): J Gras et al., Eur J Pharmacol 410:53–59 (2000) | ||||
| Amlodipine | Anti-hypertensive | − | − | (h): K Porthan et al., Ann Med 41:29–37 (2009). |
| (d): M Fujisawa et al., J Cardiovasc Pharmacol 53:325–32 (2009) | ||||
| Amoxicillin | Antibiotic | − | − | (h): T Omata et al., J Pharmacol Sci 99:531–541 (2005) |
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–71 (2005) | ||||
| Amrinone | Positive Inotrope | − | − | (h): GV Naccarelli et al., Am J Cardiol 54:600–4 (1984) |
| (d): G Onuaguluchi et al., Arch Int Pharmacodyn Ther 264:263–73 (1983) | ||||
| Aspirin | Analgesic | − | − | (h): AM Tonkin Aust N Z J Med 22:631–635 (1992). |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| (r): LM Hondeghem & P Hoffman J Cardiovasc Pharmacol 41:14–24 (2003). | ||||
| Captopril | Anti-hypertensive | − | − | (h): T Omata et al., J Pharmacol Sci 99:531–541 (2005) |
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–71 (2005) | ||||
| Ceterizine | Antihistamine | − | − | (h): R Hulhoven et al., Eur J Clin Pharmacol 63:1011–1017 (2007). |
| (d): J Weissenburger et al., Clin Exp Allergy 29(Suppl 3):190–196 (1999). | ||||
| Cilnidipine | Anti-hypertensive | − | − | (h): N Ashizawa et al., International Society for Hypertension (ISH) – Hypertension Sydney Abstract 468 (2012). |
| (d): A Takahara et al., Br J Pharmacol 158:1366–1374 (2009). | ||||
| Ciprofloxacin | Antibiotic | − | − | (h): JP Tsikouris et al. Ann Noninvasive Electrocardiol 11:52–56 (2006). |
| (d): S Toyoshima et al. J Pharmacol Sci 99:459–471 (2005) | ||||
| Enalapril | Anti-hypertensive | − | − | (h): FJ Seara et al., Ann Noninvasive Electrocardiol 8:47–54 (2003) |
| (g): P Hess et al., Lab Anim 41:470–80 (2007) | ||||
| Enalaprilat | Anti-hypertensive | − | − | (h): H Bonnemeier et al., Pacing Clin Electrophysiol 30:631–7 (2007) |
| (r): A Kijtawornrat et al., J Pharmacol Toxicol Methods 53:168–73 (2006) | ||||
| Ibandronic acid | Osteoporosis | − | − | (h): BONIVA™ (ibandronate sodium) FDA Clinical Pharmacology & Biopharmaceutics Review (NDA 21–858), 2005 |
| (d): BONIVA™ (ibandronate sodium) FDA Clinical Pharmacology & Biopharmaceutics Review (NDA 21–858), 2005 | ||||
| Mefloquine | Anti-malarial | − | − | (h): M Bindschedler et al., Eur J Clin Pharmacol 56:375–81 (2000) |
| (r): ID Lightbown et al., Br J Pharmacol 132:197–204 (2001) | ||||
| Nifedipine | Anti-hypertensive | − | + | (h): L Alberio et al., Schweiz Med Wochenschr 122:1723–7 (1992) |
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–471 (2005) | ||||
| Propranolol | Anti-hypertensive | − | − | (h): PE Puddu et al. Br Heart J 44:604–605 (1980). |
| (d): LA Hanson et al., J Pharmacol Toxicol Meth 54:116–29 (2006) | ||||
| Verapamil | Anti-hypertensive | − | + | (h): JL Holtzman et al., Clin Pharmacol Ther 46:26–32 (1989) |
| (d): S Toyoshima et al., J Pharmacol Sci 99:459–471 (2005) |
The reference corresponds to a representative QTc study conducted in humans (h), primates (m), dogs (d), rabbits (r), guinea pigs (g).
Discussion
This retrospective literature-based review examined the relationship between non-clinical (non-rodent animal) and human QT interval responses to a variety of drugs to determine whether non-clinical findings could accurately predict human cardiovascular reactions to new pharmaceuticals. It appears that there is excellent concordance between human and animal findings for drugs that increase or do not alter the QT interval. Ninety-one percent of the drugs that prolong the QT interval in humans also did so in animals (guinea pigs, rabbits, dogs, non-human primates), and 88% of the drugs that did not prolong the QT interval in humans also did not prolong the interval in animals. Our search found that non-clinical QT assays have an overall accuracy to predict human responses 90% of the time. Only four drugs (10%) in the review produced different QT interval responses in humans and animals, and these discrepancies have reasonable explanations (see below).
The need to determine whether non-clinical QT assays in animals can predict whether or not a drug will prolong the QT interval in humans has always been essential, and has increased since regulatory guidance now recommends specific non-clinical and clinical approaches to QT prolongation risk evaluation (Anonymous, 2005a,b,; Pugsley et al., 2008; Trepakova et al., 2009). Prior to the implementation of the ICH S7B and E14 regulatory guidance documents on QT assessment, other retrospective analyses of the literature were performed to determine whether safety studies of human cardiovascular drugs performed in animals and general toxicology study findings could predict human QT prolongation (Davis, 1998; De Ponti et al., 2001; Redfern et al., 2003). These analyses typically focused on drugs known to cause profound QT prolongation and TdP in human subjects. For instance, Davis (1998) gave a high-level report on nine non-cardiac medications (astemizole, cisapride, erythromycin, probucol, risperidone, sertindole, sparfloxacin, terfenadine and terodiline) approved for human use, which were found to cause QT prolongation consistently in telemetered dog and monkey safety studies. Davis (1998) only identified non-clinical reports of concordance for each drug, and did not assess models or integrate plasma drug levels to account for exposure difference across species. No examples of discordance were identified, so the animal models used were accurate predictors of human QT behaviour for this short list of high-risk drugs. De Ponti et al. (2001) used Medline to assemble an extensive list of drugs associated with clinical QT prolongation and TdP. Their analysis included literature-based references to in vitro and in vivo non-clinical reports of delayed repolarization (IKr/hERG potency and QT prolongation in an animal model), but no attempt was made to assess the concordance between clinical and non-clinical QT findings. The Redfern report (2003) was a broader review of the literature that focused on 52 drugs with a range of QT liability including very low QT and TdP risk. A feature of that analysis was the inclusion of animal and human exposure data on drug concentrations that cause hERG blockade and QT prolongation in vivo.
Identification of known QT-prolonging drugs with the text-mining approach
The current literature survey identified approximately 1600 drugs (or chemicals) that contained a reference to a QT interval (or delayed cardiac repolarization, ventricular arrhythmia or TdP) that was affected or not affected by a drug administered to a human or animal subject. This was an unexpectedly large number of citations, given prior publications that generated lists of drugs associated with QT prolongation and TdP. Three of these reports (De Ponti et al., 2001; Shah, 2002; Redfern et al., 2003) are often cited in the literature, and many of the drugs in those publications were detected by text mining. Of the 118 drugs reported by Shah, 101 (86%) were identified in the current analysis (missed drugs: acodazole, amsulalol, arteether, N-acetyl-proacainamide, butriptyline, chlorprothixene, dothiepin, D0870, fendiline, nifenalol, triethylperazine, trifluoperidol, penfluridol, pipamperone, S9788, thiothixene and tiapride). The text-mining approach identified 96% (132 of 137) of the drugs cited by De Ponti (missed drugs: clindamycin, dexfenfluramine, fenoxedil, pyrilamine, tiapride) and 100% of the drugs evaluated by Redfern. This indicates that the text search strategy can evaluate a large volume of literature and was able to detect agents that prolong QT intervals through direct (e.g. terfenadine, cisparide, haloperidol) and indirect (e.g. arsenic, pentamidine, citric acid) mechanisms of action on cardiac repolarization. The small number of missed drugs could reflect the error rate associated with text-mining approaches in general, the error rate associated with the analysis product (SIP), or the differences in the QT data sources and search strategies previously used (De Ponti et al., 2001; Shah, 2002).
Examples of QT discordance
The analysis identified four examples of discordance between human and animal studies. Diphenhydramine is a widely used antihistamine that weakly inhibits the hERG channel but can produce TdP (Woosley, 1996; Khalifa et al., 1999). Doses of diphenhydramine greater than 500 mg are associated with QT interval prolongation but not TdP in humans (Zareba et al., 1997), and do not prolong the QT interval in guinea pigs and dogs (Table 2013, Supporting Information Table S1). This discordant finding is likely related to the high doses of the drug given to human study subjects that are not reproduced in animal trials, but we lack drug exposure data to assess the difference.
A similar scenario could explain the differences in QT interval prolongation observed in human and animal models after famotidine exposure. Famotidine, which at therapeutic doses is associated with QT prolongation and TdP in humans, tests negative in animal models. The basis for this difference is unknown, but a metabolite may be responsible for QT interval prolongation, especially given the profile of this drug in hERG function and trafficking assays (Sugiyama et al., 2003; Nakamura et al., 2009; Supporting Information Table S1).
An example of a drug with low QT interval prolongation risk in humans and a false positive signal in animals was nifedipine, an L-type calcium channel blocker that is a potent arterial vasodilator and is used to treat hypertension and angina. Since the formula for QT interval correction is affected when the heart rate is elevated or reduced (Matsunaga et al., 1997; Raunig et al., 2001), it is possible that the QT interval prolongation observed in dogs following a 3 mg·kg−1 dose of nifedipine was caused by the concomitant reflex tachycardia (Toyoshima et al., 2005). This hypothesis is supported by the findings that nifedipine had no inhibitory effect on hERG current or channel trafficking at supra-therapeutic concentrations in vitro (Supporting Information Table S1).
The confounding influence of heart rate elevation on QT correction may also contribute to the complex QT effects (increase and decrease) observed with verapamil in dogs (Toyoshima et al., 2005). Verapamil is an equipotent antagonist of both hERG and L-type calcium channels (Zhang et al., 1999) and this ‘mixed channel pharmacology’, which can cause coincident prolongation and shortening of the QT interval, is thought to be the reason why QT prolongation and TdP are not observed clinically at therapeutic exposures. In the dog telemetry model, the onset of reflex tachycardia (in response to hypotension caused by vascular calcium channel blockade) also confounds the interpretation of QT interval changes (Fridericia's formula) seen following verapamil administration (Toyoshima et al., 2005). It should be noted that verapamil increased the QT interval in dogs, but only when the Bazett correction was used (Hanson et al., 2006).
Limitations of the text-mining approach
The text mining-based literature search effectively identified many QT interval prolonging drugs that had been previously identified in similar searches of humans and non-clinical models (De Ponti et al., 2001; Shah, 2002; Redfern et al., 2003; Omata et al., 2005). We believe that this literature search approach was comprehensive, but recognize that it may have imperfectly detected all the drugs associated with QT prolongation in human studies.
Despite the use of extensive text-mining vocabularies, the ability of text mining to detect key words or terms and relationships in complex sentences with large separations between primary search terms, or studies that use non-standard keyword synonyms not seen in clinical QT reports means that some drugs will go undetected. Published reports that lack a specific species in the document metadata could also account for the failure of a drug to meet inclusion criteria.
A challenge of any literature analysis is whether ‘all the relevant data’ has been cited and captured appropriately. As indicated by De Ponti et al. (2001), it may be valuable to create a registry of known drugs that have been studied for their QT liability in humans, along with relevant non-clinical evidence that pertains to QT risk assessment. Such a registry would be accessible to all parties interested in QT liability assessment and would increase our understanding of in vitro and in vivo non-clinical cardiac repolarization models. The TQT database developed at the FDA, in partnership with the HESI Pro-AWG, could be the framework for creating such a registry (Trepakova et al., 2009; Pierson et al., 2013).
Limitations of the concordance analysis
A significant limitation of the current work is that exposure data in humans and animals was not integrated into the concordance analysis because plasma drug concentrations (Cmax) were not cited in the literature reviewed. As a result, the relationship between drug exposure (total and free) across species and models is uncertain. The evaluation of drug exposure data, especially free drug concentrations, is invaluable for understanding the relationship between drug levels and the degree of hERG blockade (Supporting Information Table S1) and QT interval prolongation in vivo, and helps develop a quantitative assessment of concordance based on exposure parameters (Redfern et al., 2003).
Gathering pharmacokinetic data for the parent molecule as well as determining the contribution of metabolites to observed QT interval prolongations, would require a separate effort and was beyond the scope of this particular QT concordance exercise. This is a key limitation that reduces the current analysis to a binary assessment of the presence or absence of a QT interval prolongation signal. Therefore, any statements about QT interval concordance between clinical and non-clinical models are qualitative assessments and come from the agreement between human and animal QT interval studies.
Another caveat of this analysis is the focus on QT interval measurements as a surrogate for TdP. Other parameters, such as T-wave morphology changes or markers of T-wave dispersion of repolarization (Tpeak-Tend) were not considered, but might have been a valuable addition to the assessment. Lastly, there is the potential for publication bias, which is inherent in any literature evaluation.
Conclusions
This literature review used a text-mining approach to identify drugs that affected or did not affect the QT interval in both humans and animals. There was a 90% agreement (overall accuracy) between the animal and human findings for the drugs identified. The key message is that evaluation of new drugs in non-rodent animal cardiovascular models can be used to predict human QT interval prolongation risk. The overall findings from this literature analysis are in complete agreement with a recent cross-company data-sharing initiative, which demonstrated that the conscious dog telemetry model was valuable for predicting QT outcomes in a phase 1 clinical study (Ewart et al., 2014; n = 113 small molecules in dataset). Investigators should be aware, however, that QT prolongation can be the result of non-hERG channel-mediated mechanisms, indirect changes in autonomic nerve tone into the heart, altered channel density or properties, heart rate over-correction, and the emergence of metabolites. The underlying mechanisms of a QT interval result must be understood before QT risk can be accurately assessed.
Acknowledgments
The authors wish to express their thanks to our respective organizations and the HESI-ILSI Pro-Arrhythmia Working Group members for supporting our efforts on this collaborative project. We also thank Jane Z. Reed at INSTEM (formerly Biowisdom) for assistance with the SIP and Sophia™ systems. Lastly, we especially thank Barbara Berman for invaluable assistance with the revision process.
Glossary
- AERS
Adverse Events Reporting System
- FDA
Food and Drug Administration
- FN
false negative
- FP
false positive
- hERG
human ether-a-go-go related gene
- HESI
Health and Environmental Sciences Institute
- ICH
International Conference on Harmonization
- NHP
non-human primate
- Pro-AWG
Cardiovascular Pro-Arrhythmia Working Group
- SIP
Safety Intelligence Program
- TdP
torsade de pointes
- TN
true negative
- TP
true positive
- TQT
thorough QT/QTc study described by ICH E14
Conflict of interest
With the exception of Syril Pettit and John Koerner, all the authors are employed in the pharmaceutical industry (at the time this project was undertaken) and work for companies that sponsor the HESI Pro-Arrhythmia Project. No information presented in this paper that advocates for or promotes commercial products from any of our organizations.
Disclaimer
This publication reflects the views of the authors and does not represent views or policies of the any organization, including the FDA.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Inhibitory potency values in the hERG function and trafficking assays.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Hombo T, Kanno A, Ikeda H, Imaizumi M, Shimizu N, et al. QT PRODACT: in vivo QT assay with a conscious monkey for assessment of the potential for drug-induced QT interval prolongation. J Pharmacol Sci. 2005;99:487–500. doi: 10.1254/jphs.qt-a4. [DOI] [PubMed] [Google Scholar]
- Anonymous International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist. 2005a;70:61134–61135. [PubMed] [Google Scholar]
- Anonymous International Conference on Harmonisation; guidance on S7b Nonclinical evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist. 2005b;70:61133–61134. [PubMed] [Google Scholar]
- Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010;159:49–57. doi: 10.1111/j.1476-5381.2009.00487.x. . doi: 10.1111/j.1476-5381.2009.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AS. The pre-clinical assessment of QT interval prolongation: a comparison of in vitro and in vivo methods. Hum Exp Toxicol. 1998;17:677–680. doi: 10.1177/096032719801701205. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of torsades de pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- Ewart L, Aylott M, Deurinck M, Engwall M, Gallacher DG, Geys H, et al. The concordance between nonclinical and phase I clinical cardiovascular assessment from a cross-company data sharing initiative. Toxicol Sci. 2014;142:427–435. doi: 10.1093/toxsci/kfu198. [DOI] [PubMed] [Google Scholar]
- Fourches D, Barnes JC, Day NC, Bradley P, Reed JZ, Tropsha A. Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species. Chem Res Toxicol. 2010;23:171–183. doi: 10.1021/tx900326k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintant G. An evaluation of herg current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129:109–119. doi: 10.1016/j.pharmthera.2010.08.008. . doi: 10.1016/j.pharmthera.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Guth BD. Preclinical cardiovascular risk assessment in modern drug development. Toxicol Sci. 2007;97:4–20. doi: 10.1093/toxsci/kfm026. . doi: 10.1093/toxsci/kfm026. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Bass AS, Gintant G, Mittelstadt S, Rampe D, Thomas K. ILSI-HESI cardiovascular safety subcommittee initiative: evaluation of three non-clinical models of QT prolongation. J Pharmacol Toxicol Methods. 2006;54:116–129. doi: 10.1016/j.vascn.2006.05.001. . doi: 10.1016/j.vascn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Khalifa M, Drolet B, Daleau P, Lefez C, Gilbert M, Plante S, et al. Block of potassium currents in guinea pig ventricular myocytes and lengthening of cardiac repolarization in man by the histamine h1 receptor antagonist diphenhydramine. J Pharmacol Exp Ther. 1999;288:858–865. [PubMed] [Google Scholar]
- Matsunaga T, Mitsui T, Harada T, Inokuna M, Murano H, Shibutani Y. QT corrected for heart rate and relation between QT and RR intervals in beagle dogs. J Pharmacol Toxicol Methods. 1997;38:201–209. doi: 10.1016/s1056-8719(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Takahara A, Sugiyama A. Famotidine neither affects action potential parameters nor inhibits human ether-a-Go-Go-Related Gene (hERG) K+ current. J Toxicol Sci. 2009;34:563–567. doi: 10.2131/jts.34.563. [DOI] [PubMed] [Google Scholar]
- Newbold P, Sanders N, Reele SB. Lack of correlation between exercise and sibenadet-induced changes in heart rate corrected measurement of the QT interval. Br J Pharmacol. 2007;63:279–287. doi: 10.1111/j.1365-2125.2006.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Kasai C, Hashimoto M, Hombo T, Yamamoto K. QT PRODACT: comparison of non-clinical studies for drug-induced delay in ventricular repolarization and their role in safety evaluation in humans. J Pharmacol Sci. 2005;99:531–541. doi: 10.1254/jphs.qt-c12. [DOI] [PubMed] [Google Scholar]
- Park E, Willard J, Bi D, Fiszman M, Kozeli D, Koerner J. The impact of drug-related QT prolongation on FDA regulatory decisions. Int J Cardiol. 2013;168:4975–4976. doi: 10.1016/j.ijcard.2013.07.136. . doi: 10.1016/j.ijcard.2013.07.136. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson JB, Berridge BR, Brooks MB, Dreher K, Koerner J, Schultze AE, et al. A public-private consortium advances cardiac safety evaluation: achievements of the HESI cardiac safety technical committee. J Pharmacol Toxicol Methods. 2013;68:7–12. doi: 10.1016/j.vascn.2013.03.008. . doi: 10.1016/j.vascn.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Pollard CE, Abi Gerges N, Bridgland-Taylor MH, Easter A, Hammond TG, Valentin JP. An introduction to QT interval prolongation and non-clinical approaches to assessing and reducing risk. Br J Pharmacol. 2010;159:12–21. doi: 10.1111/j.1476-5381.2009.00207.x. . doi: 10.1111/j.1476-5381.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley MK, Authier S, Curtis MJ. Principles of safety pharmacology. Br J Pharmacol. 2008;154:1382–1399. doi: 10.1038/bjp.2008.280. . doi: 10.1038/bjp.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunig D, Depasquale MJ, Huang CH, Winslow R, Fossa AA. Statistical analysis of Qt interval as a function of changes in Rr interval in the conscious dog. J Pharmacol Toxicol Methods. 2001;46:1–11. doi: 10.1016/s1056-8719(01)00158-7. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Shah RR. Drug-induced prolongation of the QT interval: regulatory dilemmas and implications for approval and labelling of a new chemical entity. Fundam Clin Pharmacol. 2002;16:147–156. doi: 10.1046/j.1472-8206.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- Spasic I, Ananiadou S, McNaught J, Kumar A. Text mining and ontologies in biomedicine: making sense of raw text. Brief Bioinform. 2005;6:239–251. doi: 10.1093/bib/6.3.239. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Satoh Y, Takahara A, Nakamura Y, Shimizu-Sasamata M, Sato S, et al. Famotidine does not induce long QT syndrome: experimental evidence from in vitro and in vivo test systems. Eur J Pharmacol. 2003;466:137–146. doi: 10.1016/s0014-2999(03)01559-0. [DOI] [PubMed] [Google Scholar]
- Toyoshima S, Kanno A, Kitayama T, Sekiya K, Nakai K, Haruna M, et al. QT prodact: in vivo QT assay in the conscious dog for assessing the potential for QT interval prolongation by human pharmaceuticals. J Pharmacol Sci. 2005;99:459–471. doi: 10.1254/jphs.qt-a2. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Koerner J, Pettit SD, Valentin JP. A HESI consortium approach to assess the human predictive value of non-clinical repolarization assays. J Pharmacol Toxicol Methods. 2009;60:45–50. doi: 10.1016/j.vascn.2009.05.002. . doi: 10.1016/j.vascn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Valentin JP, Newbold P, Hammond TG. Third focused issue on safety pharmacology. predictive value of nonclinical models to assess QT liability in man: Sibenadet (Viozan), a case study. J Pharmacol Toxicol Methods. 2006;54:253. [Google Scholar]
- Valentin JP, Bialecki R, Ewart L, Hammond T, Leishmann D, Lindgren S, et al. A framework to assess the translation of safety pharmacology data to humans. J Pharmacol Toxicol Methods. 2009;60:152–158. doi: 10.1016/j.vascn.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Vargas HM, Bass AS, Breidenbach A, Feldman HS, Gintant GA, Harmer AR, et al. Scientific review and recommendations on preclinical cardiovascular safety evaluation of biologics. J Pharmacol Toxicol Methods. 2008;58:72–76. doi: 10.1016/j.vascn.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Vargas HM, Bass AS, Koerner J, Matis-Mitchell S, Pugsley M, Skinner M, et al. Evaluation of QTc prolongation in animal and human studies: a qualitative assessment of nonclinical and clinical concordance based on the literature. J Pharmacol Toxicol Methods. 2012;66:203. [Google Scholar]
- Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel. 2002;5:116–126. [PubMed] [Google Scholar]
- Woosley RL. Cardiac actions of antihistamines. Annu Rev Pharmacol Toxicol. 1996;36:233–252. doi: 10.1146/annurev.pa.36.040196.001313. . doi: 10.1146/annurev.pa.36.040196.001313. [DOI] [PubMed] [Google Scholar]
- Zareba W, Moss AJ, Rosero SZ, Hajj-Ali R, Konecki J, Andrews M. Electrocardiographic findings in patients with diphenhydramine overdose. Am J Cardiol. 1997;80:1168–1173. doi: 10.1016/s0002-9149(97)00634-6. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Gong Q, Makielski JC, January CT. Mechanism of block and identification of the verapamil binding domain to herg potassium channels. Circ Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Inhibitory potency values in the hERG function and trafficking assays.


