Abstract
Background and Purpose
Pelitinib is a potent irreversible EGFR TK inhibitor currently in clinical trials for the treatment of lung cancer. Hyperthermia has been applied concomitantly with chemotherapy and radiotherapy to enhance treatment outcome. In this study, we investigated the ability of the combination of pelitinib with other conventional anticancer drugs to specifically target cancer cells with up-regulated efflux transporters ABCB1/ABCG2 after hyperthermia as a novel way to eradicate the cancer stem-like cells responsible for cancer recurrence.
Experimental Approach
Alterations in intracellular topotecan accumulation, the efflux of fluorescent probe substrates, expression and ATPase activity of ABCB1/ABCG2 and tumoursphere formation capacity of side population (SP) cells sorted after hyperthermia were examined to elucidate the mechanism of pelitinib-induced chemosensitization.
Key Results
While pelitinib did not modulate ABCB1/ABCG2 expressions, the combination of pelitinib with transporter substrate anticancer drugs induced more marked apoptosis, specifically in cells exposed to hyperthermia. The flow cytometric assay showed that both ABCB1- and ABCG2-mediated drug effluxes were significantly inhibited by pelitinib in a concentration-dependent manner. The inhibition kinetics suggested that pelitinib is a competitive inhibitor of ABCB1/ABCG2, which is consistent with its ability to stimulate their ATPase activity. SP cells sorted after hyperthermia were found to be more resistant to anticancer drugs, presumably due to the up-regulation of ABCB1 and ABCG2. Importantly, pelitinib specifically enhanced the chemosensitivity but reduced the tumoursphere formation capacity of these SP cells.
Conclusions and Implications
This study demonstrated a novel approach, exploiting drug resistance, to selectively kill cancer stem-like cells after hyperthermia.
Tables of Links
| LIGANDS | |
|---|---|
| Cisplatin | Paclitaxel |
| Doxorubicin | Pelitinib |
| EGF | Rhodamine 123 |
| Insulin | Topotecan |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b,).
Introduction
The large family of ATP-binding cassette (ABC) transporters, including P-glycoprotein (ABCB1/P-gp), ABCC1/MRP1 and ABCG2, play a key role in the energy-dependent cellular efflux of chemotherapeutic drugs. They are capable of recognizing and extruding a broad range of structurally and functionally unrelated anticancer drugs, thereby leading to multidrug resistance (MDR) in cancer cells. Cancer recurrence is a major hurdle hindering successful chemotherapy. It is believed to arise from the survival of cancer stem-like cells (CSCs) after anticancer treatment. To this end, cancer stem-like phenotypes are correlated with elevated expression of the efflux transporters ABCB1 and/or ABCG2 (Ho et al., 2007), which protect the cancer cells from chemotherapy. Therefore, substantial research efforts have been made to target these CSCs to eliminate metastasis and prevent recurrence (Frank et al., 2010; Takebe et al., 2011).
TK inhibitors (TKIs) are molecularly targeted chemotherapeutic drugs that specifically inhibit several oncogenic TKs to regulate cancer proliferation, invasion, metastasis and angiogenesis. The recent discovery that TKIs can potently and specifically inhibit various ABC transporters has advocated the use of TKIs as chemosensitizers to circumvent MDR. In this regard, we have previously demonstrated that apatinib (Mi et al., 2010; Tong et al., 2012), axitinib (Wang et al., 2012), crizotinib (Zhou et al., 2012), vandetanib (Zheng et al., 2009) and volasertib (To et al., 2013) inhibit various ABC transporters and reverse MDR in leukaemia and solid tumours.
Lung cancer is one of the most common human malignancies and the leading cause of cancer-related deaths worldwide (Jemal et al., 2011). Systemic chemotherapy is an important treatment option for advanced lung cancer, but response to traditional cytotoxic chemotherapy is poor. Hyperthermia is a promising treatment strategy for metastatic lung cancer (Zwischenberger et al., 2001). Human lung cancer cells have an increased thermosensitivity when compared with normal lung cells (van der Zee, 2002). It has also been demonstrated that hyperthermia can sensitize cancer cells to subsequent chemo/radiation therapy (Dumontet et al., 1998; Mohamed et al., 2003; Schildkopf et al., 2010). Hyperthermia (40.0–43.5°C) is known to cause direct damage to cell membranes, affect the integrity of the DNA structure and alter the intracellular responses (Pietrangeli and Mondovi, 2006). In a phase I clinical trial, systemic hyperthermia has been shown to be well tolerated (Zwischenberger et al., 2004), thus making it an attractive adjuvant therapy. In this study, hyperthermia at 42.5°C was chosen as the optimal temperature for experiments. Protein denaturation under hyperthermia has been demonstrated to elicit a beneficial cell-killing effect. While there is excessive protein denaturation at temperaturse above 43°C, a sufficiently elevated temperature (chosen as 42.5°C) is necessary to facilitate the cellular response after hyperthermia (Lepock, 2003).
Interestingly, hyperthermia or heat shock is known to up-regulate some ABC transporters such as ABCB1/P-gp (Miyazaki et al., 1992; Stein et al., 2001). We found that both ABCB1 and ABCG2 are up-regulated by hyperthermia in lung cancer. Pelitinib (EKB-569) is a potent irreversible EGFR TKI recently developed and being evaluated in clinical trials for lung cancer and various other solid cancers. It is a promising new drug candidate in development because it has been shown to be effective in lung cancer bearing the secondary EGFR, T790M mutation, which is resistant to the first-generation EGFR TKIs (Kwak et al., 2005). In this study we sought to exploit the combination of pelitinib with other conventional anticancer drugs to specifically target cancer cells with up-regulated ABCB1/ABCG2 after hyperthermia as a novel way to eradicate CSCs and prevent cancer recurrence.
Methods
Cell culture
Most cell lines used in our study were generous gifts from Dr Susan Bates (National Cancer Institute), except LCC6 and its ABCB1-overepxressing subline LCC6 MDR1 (which was provided by Professor Robert Clarke, Georgetown University, Washington, DC, USA). Pairs of parental and drug-resistant sublines with overexpression of the three major MDR transporters were used in our study, which include human breast cancer LCC6/its ABCB1-overexpressing LCC6 MDR1 subline (Leonessa et al., 1996), human colon cancer S1/its ABCG2-overexpressing S1M1 80 subline (Miyake et al., 1999) and MCF-7/its ABCC1 (MRP1)-overexpressing MCF-7 VP-16 subline (Schneider et al., 1994). The human primary embryonic kidney cell line HEK293 and its stable pcDNA3-, ABCB1-, ABCC1- or ABCG2-transfected cell lines were also used to demonstrate the specific effect of pelitinib on the respective transporters. LCC6, LCC6 MDR1, MCF-7 and MCF-7 VP-16 were maintained in DMEM medium whereas S1 and S1-M1-80 were grown in MEM medium supplemented with 10% FBS, 100 u·mL−1 streptomycin sulfate and 100 u·mL−1 penicillin G sulfate, and incubated at 37°C in 5% CO2. The transfected cells were cultured in complete DMEM medium supplemented with 2 mg·mL−1 G418. The human non-small cell lung cancer (NSCLC) cell lines A549 (maintained in complete DMEM medium) and H460 (grown in complete RPMI1640 medium) were used for the hyperthermia experiments. Hyperthermia was applied for 1 or 4 h at 42.5 ± 0.2°C in a designated tissue culture incubator. The respective controls were kept at 37 ± 0.2°C.
Reverse transcription and quantitative real-time PCR
Real-time quantitative RT-PCR was performed as described previously (To and Tomlinson, 2013) to evaluate the expression of ABCB1, ABCC1 and ABCG2 in cells treated with or without pelitinib upon hyperthermia exposure.
Luciferase reporter assays
A series of luciferase reporter constructs spanning the full-length human ABCG2 promoter or its fragments with progressive deletions at the 5′ end has been previously described (To et al., 2006). Two new reporter constructs were prepared: one with a putative heat-shock element (HSE) sequence deleted from the full-length ABCG2 promoter, whereas the other one with the putative HSE mutated (Figure 1C). The ABCG2 promoter/Firefly luciferase fusion genes (200 ng DNA) were transfected in A549 cells by using lipofectamine 2000 (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. The pGL3-Basic (promoterless) plasmid, encoding Firefly luciferase (Promega, Madison, WI, USA), was used to determine the basal levels. In each experiment, the phRG-Basic plasmid (50 ng), encoding Renilla luciferase (Promega) was co-transfected for normalization purposes. To analyse the heat-inducible ABCG2 promoter-driven luciferase activity in A549 cells, transient transduction was performed using the Luc reporter constructs (Figure 1C). Cells were incubated for 48 h after transfection and then exposed to hyperthermia for 1 or 4 h at 42.5°C (or at 37°C). Luminescence was then measured at 16 h after cell recovery from the hyperthermia treatment by the dual luciferase reporter assay system (Promega). Reporter activity was normalized by calculating the ratio of Firefly/Renilla values. Results are expressed as mean ± SD of duplicate measurements from three independent transfections.
Figure 1.
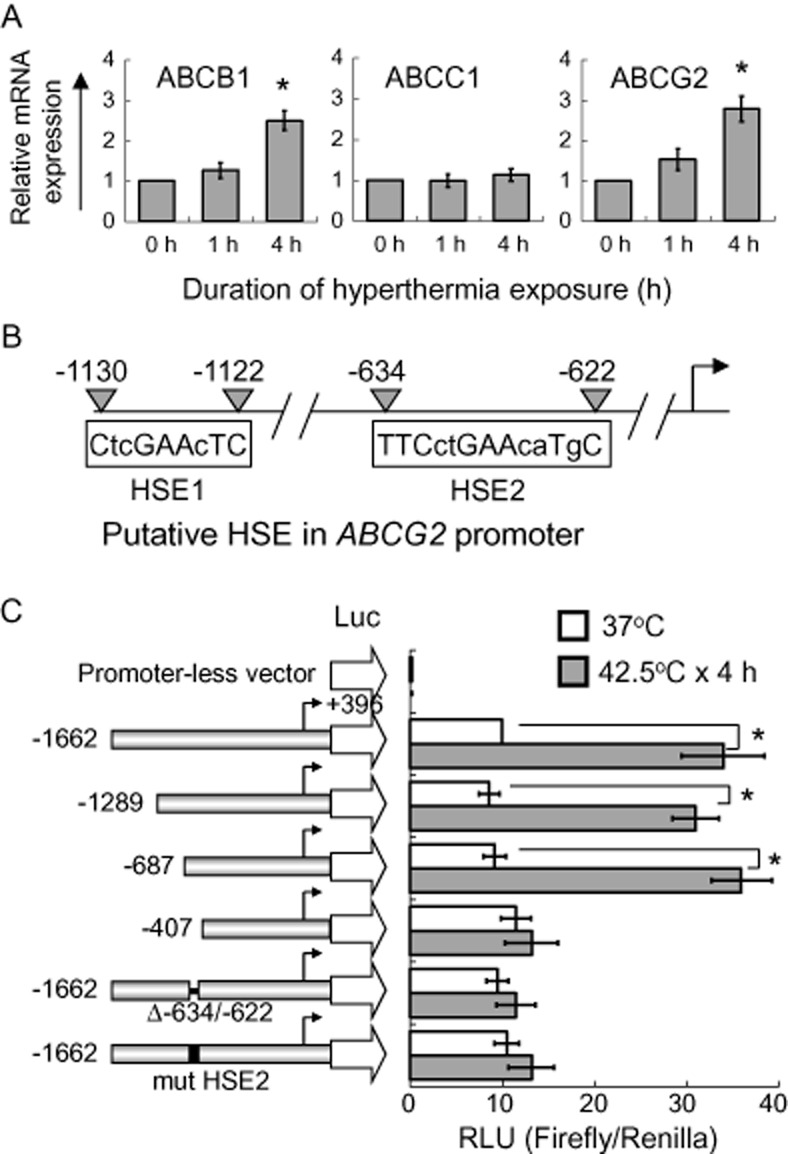
Up-regulation of ABCB1 and ABCG2 by hyperthermia in A549 cells. (A) Real-time PCR analysis of ABCB1, ABCC1 and ABCG2 mRNA expression in A549 with or without hyperthermia treatment at 42.5°C for 1 or 4 h. Relative ABCB1, ABCC1 or ABCG2 transcript expression is shown after normalization with GAPDH. *P < 0.05, compared with cell culture at 37°C. (B) Schematic illustration of two putative HSEs (HSE1 and HSE2) in the ABCG2 promoter. The upper case letters represent the nucleotides matching the reported human consensus HSE sequence (TTCnnGAAnnTTC; n = any nucleotide; Akerfelt et al., 2010). (C) ABCG2 promoter luciferase reporter assay. Left, schematic representation of the 5′-deletion ABCG2 promoter constructs. The 5′-end of each of the constructs relative to the transcription start site (arrows) is indicated. The pGL3-basic (promoterless) vector, encoding firefly luciferase, was used to determine the basal levels. The construct (Δ-634/-622) was prepared with the putative HSE2 deleted from the full-length ABCG2 promoter, whereas the other construct (mut HSE2) was prepared with the putative HSE2 mutated from TTCctGAAcaTgC to AGTctAGCctGgA. Right, ABCG2 transcriptional activity in A549 cells transiently transfected with the various ABCG2 promoter constructs with or without hyperthermia treatment at 42.5°C for 4 h. The mean reporter activity ± SD [Firefly/Renilla luciferase units (RLU)] from three independent experiments is presented. *P < 0.05, compared with cell culture at 37°C.
Growth inhibition assay
The growth inhibitory effect of individual anticancer drugs was evaluated by the sulforhodamine B assay as described previously (Skehan et al., 1990). For the combination drug treatment, drugs were given simultaneously for 72 h.
Analysis of cell surface expression of ABCB1 and ABCG2 by flow cytometry
Cell surface ABCB1 and ABCG2 expression with or without hyperthermia exposure were determined by flow cytometry as described previously (To and Tomlinson, 2013). Briefly, A549 cells with or without hyperthermia exposure (4 h) were fixed with 4% paraformaldehyde for 10 min, washed and then labelled with either phycoerythrin (PE)-conjugated anti-ABCB1, anti-ABCG2 antibodies or PE-conjugated mouse IgG2b negative control antibody (eBioscience; San Diego, CA, USA) in 2% BSA/Dulbecco's PBS at 37°C for 30 min. Labelled cells were washed in PBS and assayed by flow cytometry on a LSRFortessa cell analyser (BD Biosciences, San Jose, CA, USA). PE fluorescence was detected with a 488 nm argon laser and a 585 nm band-pass filter.
Flow cytometric analysis of transporter activity using fluorescent probe substrates
A flow cytometry-based assay was employed to study the inhibition of transport activity of the three major MDR transporters (ABCB1, ABCC1 and ABCG2) by pelitinib in the respective transporter-stably transfected HEK293 cells as described previously (To and Tomlinson, 2013). Fluorescent probe substrates (0.5 μg·mL−1 Rh123, 1 μM PhA or 0.2 μM calcein AM) specific for the three transporters were used. Inhibitors specific for ABCB1 (PSC833), ABCC1 (MK571) and ABCG2 (FTC) were used as control for comparison. Samples were analysed using LSRFortessa cell analyser (BD Biosciences).
Analysis of ABCB1 and ABCG2 inhibition kinetics
The inhibition kinetics of ABCB1- or ABCG2-mediated efflux of topotecan by pelitinib was evaluated as described previously in the transporter-stably transfected cells with minor modification (To and Tomlinson, 2013). Topotecan was chosen because it is a substrate of both ABCB1 and ABCG2. The drug is included in most combination regimens for lung cancer treatment (O'Brien et al., 2007). Briefly, ABCB1- or ABCG2-stably transfected HEK293 cells were incubated with various concentration of topotecan (1, 2, 5 and 10 μM) in the presence of four designated concentrations of pelitinib (0, 1, 2 or 5 μM) for 3 h at 37°C. The cells were then collected, centrifuged and washed once with cold PBS and resuspended in the medium without topotecan in the absence or presence of pelitinib. Subsequently, cells were incubated for 10 min at 37°C to allow for efflux, centrifuged and washed three times with ice-cold PBS. In the control samples, the incubations were kept at 0°C. Finally, the intracellular concentration of topotecan was determined by flow cytometric analysis as mentioned earlier. Topotecan fluorescence was measured with a 355 UV laser and a 530/30 nm band-pass filter. The quantity of topotecan efflux by ABCB1 or ABCG2 was calculated by subtracting the values obtained at 37°C from that at 0°C in the respective transporter-stably transfected cells. The inhibitory effect of pelitinib on the two transporters was then analysed by the Lineweaver–Burk plot.
Cellular accumulation of topotecan in A549 cells
The effect of hyperthermia on the accumulation of topotecan (substrate of both ABCB1 and ABCG2) in A549 cells was determined by fluorescence microscopy and flow cytometry. The cells were incubated at 37 or 42.5°C for 4 h before exposure to 10 μM of topotecan for 30 min. The cells were then washed twice with ice-cold PBS. Cellular accumulation of topotecan was observed under a Nikon TE2000U fluorescence inverted microscope (Nikon Precision, Belmont, CA, USA). For flow cytometry, the cells were then collected and analysed as mentioned earlier.
Transporter ATPase assay
The effect of pelitinib on the vanadate-sensitive ATPase activity of ABCB1 or ABCG2 in cell membrane prepared from High-Five insect cells was measured using the BD Gentest ATPase assay kit (BD Biosciences) according to the manufacturer's instructions.
Apoptosis assay
A549 cells were grown on 60 mm tissue culture dish at a density of about 2.0 × 105 cells per well. After treatment with 20 nM topotecan in the presence or absence of 3 μM pelitinib for 48 h, both floating and attached cells were collected and washed twice with ice-cold PBS. The proportion of apoptotic cells was determined by using the APC Annexin V Apoptosis Kit (BD Bioscience) according to the manufacturer's instruction.
Side population (SP) sorting and analysis
Cell suspensions were labelled with Hoechst 33342 dye as described by Goodell et al. (1997) with minor modifications. Briefly, A549 cells were resuspended at 1 × 106·mL−1 in pre-warmed DMEM with 2% FBS and 10 mM HEPES buffer. Hoechst 33342 dye was added at a final concentration of 5 μg·mL−1 in the presence or absence of 1 μM tariquidar. Tariquidar was used because it is known to inhibit both ABCB1 and ABCG2, the two major ABC transporters responsible for the SP phenotype. The cells were then incubated at 37°C for 90 min with intermittent shaking. At the end of the incubation, the cells were washed with ice-cold PBS, centrifuged at 4°C and resuspended in ice-cold PBS. Propidium iodide at a final concentration of 40 μg·mL−1 was added to the cells to indicate the viable cells. Analyses and sorting were performed on a BD FACSAria II Cell Sorter (BD Biosciences). The Hoechst 33342 dye was excited with a near UV laser at 375 nm, emissions of Hoechst 33342 were detected using 660/20 nm (Hoechst red) and 450/40 nm (Hoechst blue) band-pass filters. Sorted SP and non-SP cells were collected and incubated in complete DMEM medium supplemented with 10 mM HEPES for a further drug efflux assay or apoptosis assay.
Tumoursphere formation assay
The SP and NSP cells sorted from A549, with or without hyperthermia exposure (42.5°C for 4 h), were treated with topotecan 2 nM in the presence or absence of pelitinib 3 μM for 48 h. The cells were then resuspended and cultured in serum-free DMEM medium supplemented with 20 ng·mL−1 of EGF (Life Technologies), 20 ng·mL−1 of basic fibroblast growth factor (bFGF) (Life Technologies) and 200 ng·mL−1 of insulin (Sigma) on flat bottom ultra-low attachment multiwell plates (Corning Life Sciences, Manassas, VA, USA). The cells were allowed to grow for 12 days and the number of tumourspheres with a diameter of > 20 μm was counted.
Statistical analysis
All experiments were repeated at least three times. The statistical software SPSS16.0 (IBM, Armonk, NY, USA) was used for data analysis. Statistical significance was determined at P < 0.05 by Student's t-test.
Chemicals and reagents
Rhodamine 123 (Rh123) and topotecan were obtained from Sigma Chemical (St Louis, MO, USA). Fumitremorgin C (FTC), Ko143, pheophorbide A (PhA), PSC833 and tariquidar were kind gifts provided by Dr Susan Bates (National Cancer Institute, NIH, Bethesda, MD, USA).
Results
ABCB1 and ABCG2 were up-regulated in lung cancer cells after hyperthermia exposure
The three most important MDR transporters (ABCB1, ABCC1 and ABCG2) were evaluated for their potential up-regulation after hyperthermia exposure in lung cancer cell lines A549, H460 (both harbouring wild-type EGFRs) and H1975 (harbouring the secondary EGFR T790M mutation). After 4 h of hyperthermia exposure (42.5°C), both ABCB1 and ABCG2 mRNA expressions were increased significantly about three times in A549 (P < 0.05; Figure 1A). The up-regulation of the two transporters was found to be temperature-dependent (a gradual increase from 39 to 42.5° C; Supporting Information Fig. S1). A significant increase of about 2.5 times of the two transporters was also observed in two other NSCLC cell lines H460 and H1975 (P < 0.05; Supporting Information Fig. S1). In contrast, ABCC1 expression was not altered in any of the cell lines tested. Consistent with mRNA expression, cell surface staining and total protein expression of ABCB1 and ABCG2 were also increased significantly by ∼2 times (ABCB1) and ∼3 times (ABCG2) in A549 after 4 h of hyperthermia exposure (P < 0.05; Figure 2A and B). A similar increase in cell surface staining and total protein expression of ABCB1 and ABCG2 was also observed in another cell line H1975 (Supporting Information Fig. S2A and B).
Figure 2.
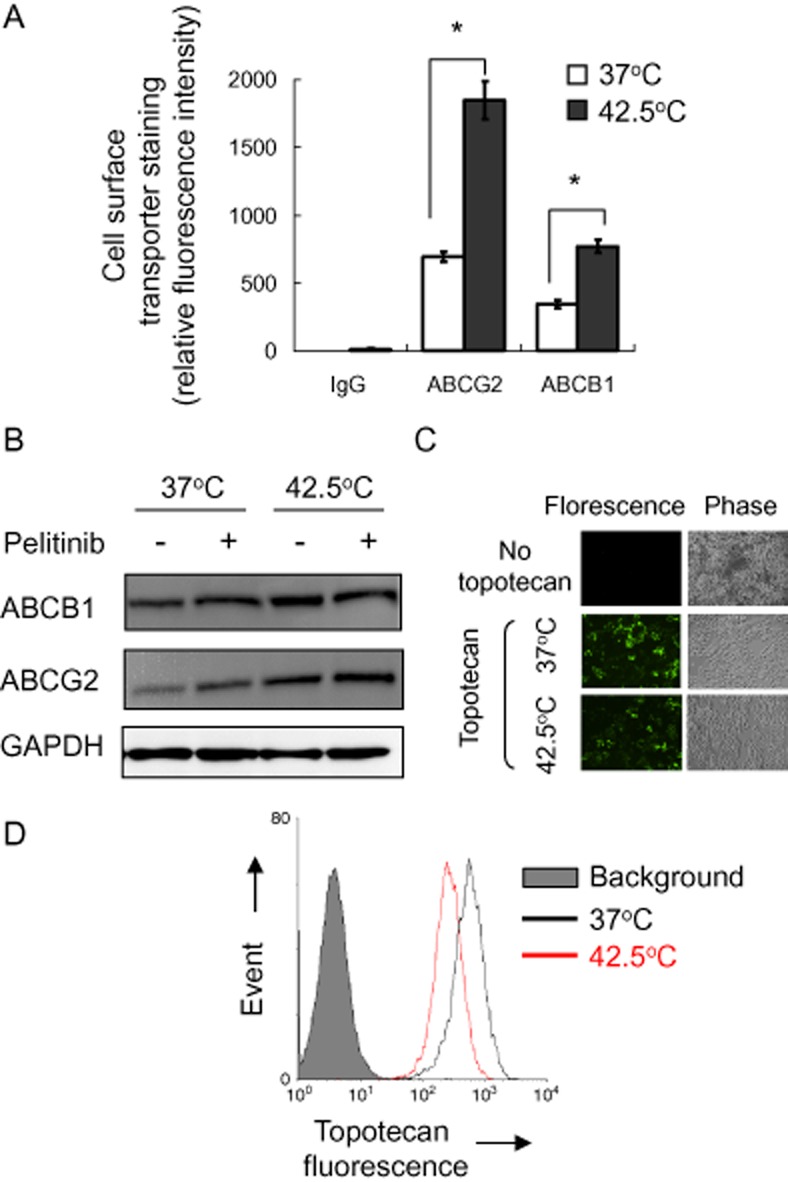
Cell surface expression and functional analysis of ABCG2 and ABCB1 in A549 cells with or without hyperthermia (42.5°C) treatment. (A) Cell surface ABCG2 or ABCB1 staining of A549 cells with or without hyperthermia (42.5°C) pretreatment was quantified by subtracting the fluorescence signal from 5D3 or UIC2 labelling by that from the control IgG isotype antibody labelling. Mean ± SD from three independent experiments is shown. *P < 0.05, compared with cell culture at 37°C. (B) Western blot analysis of total ABCB1 or ABCG2 protein expression in A549 cells with or without 4 h of hyperthermia (42.5°C) treatment. It is also noted that pelitinib treatment did not alter the up-regulated ABCB1 or ABCG2 protein expression after hyperthermia (42.5°C) treatment. (C) Fluorescence microscopy images showing the steady state accumulation of a fluorescent ABCG2/ABCB1 substrate anticancer drug topotecan (drug incubation at 37°C × 60 min) in A549 cells with or without 4 h of hyperthermia pretreatment. Left panel, fluorescence image; right panel, phase contrast image from the corresponding field of view. (D) Decreased cellular accumulation of topotecan in A549 cells after hyperthermia as detected by flow cytometry. Drug incubation and hyperthermia treatment were the same as in (C). After the drug incubation, the cells were collected, washed twice in ice-cold PBS and retention of the fluorescence inside the cells was analysed by flow cytometry. Flow cytometry histogram from a representative experiment is shown.
While the transcriptional up-regulation of ABCB1 by hyperthermia has been reported and a heat shock element (HSE) on ABCB1 promoter has been identified (Miyazaki et al., 1992), the regulation of ABCG2 by elevated temperature is not clear. Therefore, the induction of ABCG2 after hyperthermia was further examined using a reporter gene assay. To this end, two putative HSEs (HSE1 and HSE2) were identified in the ABCG2 promoter (Figure 1B), which resemble the consensus HSE sequence reported for other heat shock-responsive genes (Akerfelt et al., 2010). A series of reporter gene constructs spanning the ABCG2 promoter were employed to verify whether the two putative HSEs are responsible for the observed hyperthermia-induced ABCG2 in A549 cells (Figure 1C). After transiently transfected into A549 cells, all of the ABCG2 promoter luciferase constructs exhibited good activities above the promoterless pGL3-Basic background (Figure 1C). While reporter activity of the full-length ABCG2 promoter construct (−1662/+396) and two other long fragments (−1289 and −628/+396) were found to be significantly up-regulated ∼3 times after hyperthermia exposure (42.5°C × 4 h; P < 0.05), the induction was abolished in a shorter ABCG2 promoter construct (−407/+396) and in the full-length ABCG2 promoter constructs with the putative HSE2 binding sequence deleted (i.e. Δ-634/-622) or mutated (i.e. mut HSE2) (Figure 1C).
Cellular accumulation of anticancer drug was decreased after hyperthermia exposure
As both ABCB1 and ABCG2 were induced by hyperthermia, the cellular accumulation of anticancer drugs may be affected. Topotecan, a substrate of both ABCB1 and ABCG2, was chosen as a model anticancer drug for evaluation. By fluorescence microscopy and flow cytometric assay, the intracellular accumulation of topotecan in A549 cells was found to be decreased significantly by 18 ± 5% (flow cytometry; P < 0.05) after hyperthermia exposure (Figure 2C and D). A similar decrease in topotecan accumulation was also observed in another NSCLC cell line H1975 (decreased by 13 ± 4%; flow cytometry) after 4 h of hyperthermia exposure (Supporting Information Fig. S2C). Therefore, the decreased drug accumulation after hyperthermia may be a general phenomenon.
The decreased anticancer drug accumulation after hyperthermia may therefore limit the therapeutic response in cancer cells. Anticancer activity of topotecan and etoposide was then evaluated in A549 and H460 cells. Interestingly, both anticancer drugs were found to exhibit slightly greater anticancer activity in the two cell lines after hyperthermia exposure, albeit no statistical difference from the same treatment at 37°C was observed (Supporting Information Table S1).
Pelitinib inhibited the three major MDR transporters
The potent and specific inhibition of various MDR transporters by a number of molecular targeted TKIs has been recently reported. We therefore hypothesized that TKIs may be used to inhibit the up-regulated ABCB1 and ABCG2 after hyperthermia to enhance the response to chemotherapy. In this study, a new irreversible EGF receptor TKI, pelitinib, was investigated.
Pelitinib was first evaluated for its inhibition of ABCB1, ABCC1 and ABCG2, the three most important MDR transporters. Using specific fluorescent probe substrates for the three transporters (Rh123 for ABCB1, calcein AM for ABCC1 and PhA for ABCG2), the inhibition of their efflux was evaluated in drug-resistant cancer cell lines specifically overexpressing the transporters and in stably transfected HEK293 cells (Figure 3). The read-out of the assay is the accumulation of the fluorescent probe substrate after a 1 h drug-free efflux. Inhibition of the transporter-mediated efflux is indicated by a shift to higher intracellular fluorescence signal. As illustrated in Figure 3, pelitinib was found to inhibit all three MDR transporters in a concentration-dependent manner.
Figure 3.
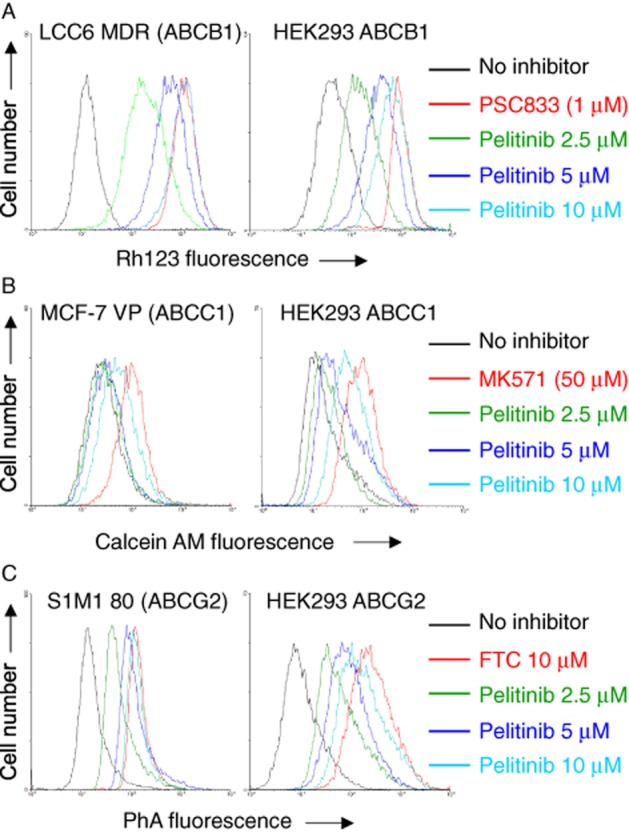
Inhibition of ABCB1-, ABCC1- or ABCG2-mediated efflux of fluorescent probe substrate by pelitinib in drug-resistant cells overexpressing the transporters (left panel) or HEK293 cells stably transfected with the three transporters (right panel). Cells were incubated with fluorescent probe for each transporter: 0.5 mg·mL−1 Rh123 alone (A), 1 μM calcein AM (B), 1 μM PhA alone (C) (black); the corresponding fluorescent probe in the presence of pelitinib at the indicated concentrations (various colours) or the corresponding fluorescent probe in the presence of specific inhibitor for the corresponding transporter (1 μM PSC833 for ABCB1, 50 μM MK571 for ABCC1 and 10 μM FTC for ABCG2) (red) at 37°C for 30 min. Retention of the fluorescent probe substrates in the cells after a 1 h substrate-free efflux was measured by flow cytometry. Representative histograms from three independent experiments are shown.
The reversal of MDR by pelitinib was then evaluated in three pairs of parental and drug-resistant cancer cell lines with known overexpression of the three individual MDR transporters. The resistant cancer cell lines are remarkably resistant to the corresponding transporter substrate anticancer drugs (Table 2010; i.e. ABCB1-overexpressing LCC6 MDR1: 112-fold resistant to paclitaxel; ABCC1-overexpressing MCF-7 VP: 29-fold resistant to doxorubicin; ABCG2-overexpressing S1M1 80: 48-fold resistant to topotecan). A panel of ABCB1-, ABCC1- or ABCG2-stably transfected HEK293 HEK cell lines was also tested, which demonstrated different extent of resistance to the transporter substrate anticancer drugs. While pelitinib did not appreciably affect cell proliferation at the highest concentration tested in all cell lines (data not shown), it was found to potentiate the anticancer activity of the transporter substrate anticancer drugs in a concentration-dependent manner in the corresponding transporter-overexpressing cell line (Table 2010). Its effect was specific because resistance reversal was not observed in the parental cells without transporter overexpression. Moreover, pelitinib did not affect the anticancer activity of cisplatin (which is not transported by the transporters tested) in all cell line models tested.
Table 1.
Effect of pelitinib on reversing ABCB1-, ABCG2- and ABCC1-mediated MDR
| Compounds | IC50 ± SD (fold resistance) | |||
|---|---|---|---|---|
| MDA435/LCC6a | MDA435/LCC6 MDRb | HEK293/pcDNA3c | HEK293/ABCB1d | |
| Paclitaxel | 1.21 ± 0.15 nM (1) | 135.4 ± 12.3 nM (112) | 9.82 ± 0.72 nM (1) | 4356.5 ± 613.1 nM (444) |
| + 400 nM PSC833 | 1.32 ± 0.16 nM (1.1) | 2.95 ± 0.42 nM (2.4)* | 9.15 ± 0.66 nM (0.9) | 23.61 ± 2.25 nM (2.4)* |
| + 0.75 μM pelitinib | 1.58 ± 0.36 nM (1.3) | 118.5 ± 24.5 nM (98) | 10.59 ± 0.56 nM (1.1) | 3865.9 ± 423.6 nM (394) |
| + 1.5 μM pelitinib | 1.19 ± 0.41 nM (1.0) | 82.6 ± 26.4 nM (68)* | 9.32 ± 0.65 nM (0.9) | 2826.5 ± 326.9 nM (288)* |
| + 3 μM pelitinib | 1.08 ± 0.31 nM (0.9) | 42.5 ± 13.9 nM (35)* | 9.05 ± 0.81 nM (0.9) | 1232.9 ± 263.7 nM (126)* |
| Cisplatin | 6.75 ± 1.81 μM (1) | 6.03 ± 0.86 μM (0.9) | 2.10 ± 0.48 μM (1) | 2.31 ± 0.35 μM (1.1) |
| + 3 μM pelitinib | 5.89 ± 1.23 μM (0.9) | 6.13 ± 0.95 μM (0.9) | 1.95 ± 0.46 μM (0.9) | 2.22 ± 0.38 μM (1.1) |
| S1 | S1M1 80 | HEK293/pcDNA3 | HEK293/ABCG2 | |
|---|---|---|---|---|
| Topotecan | 0.24 ± 0.05 nM (1) | 11.51 ± 0.48 nM (48) | 8.65 ± 1.15 nM (1) | 289.5 ± 46.3 nM (33) |
| + 2.5 μM FTC | 0.25 ± 0.02 nM (1.0) | 0.75 ± 0.06 nM (3.1)* | 7.95 ± 1.35 nM (0.9) | 29.5 ± 4.2 nM (3.4)* |
| + 0.75 μM pelitinib | 0.31 ± 0.04 nM (1.3) | 9.25 ± 0.88 nM (39) | 8.89 ± 1.56 nM (1.0) | 253.6 ± 39.5 nM (29) |
| + 1.5 μM pelitinib | 0.29 ± 0.06 nM (1.2) | 5.32 ± 0.44 nM (22)* | 7.52 ± 1.31 nM (0.9) | 112.8 ± 35.2 nM (13)* |
| + 3 μM pelitinib | 0.22 ± 0.05 nM (0.9) | 2.86 ± 0.52 nM (12)* | 8.03 ± 1.44 nM (0.9) | 72.1 ± 25.3 nM (8.3)* |
| Cisplatin | 14.16 ± 1.12 μM (1) | 13.49 ± 1.83 μM (1.0) | 2.10 ± 0.48 μM (1) | 2.21 ± 0.41 μM (1.1) |
| + 3 μM pelitinib | 13.20 ± 1.45 μM (0.9) | 14.55 ± 1.59 μM (1.0) | 1.95 ± 0.46 μM (0.9) | 1.88 ± 0.52 μM (0.9) |
| MCF-7 | MCF-7 VP | HEK293/pcDNA3 | HEK293/ABCC1 | |
|---|---|---|---|---|
| Doxorubicin | 0.34 ± 0.05 μM (1) | 9.73 ± 1.04 μM (28.6) | 73.2 ± 12.5 nM (1) | 832.5 ± 68.6 nM (11.4) |
| + 40 μM MK571 | 0.29 ± 0.02 μM (0.9) | 1.25 ± 0.31 μM (3.7)* | 65.3 ± 15.2 nM (0.9) | 155.2 ± 35.2 nM (2.1)* |
| + 0.75 μM pelitinib | 0.36 ± 0.06 μM (1.1) | 8.55 ± 1.11 μM (25.1) | 76.5 ± 13.5 nM (1.0) | 752.6 ± 48.3 nM (10.3) |
| + 1.5 μM pelitinib | 0.41 ± 0.07 μM (1.2) | 5.69 ± 0.75 μM (16.7)* | 74.8 ± 14.6 nM (1.0) | 423.7 ± 65.2 nM (5.8)* |
| + 3 μM pelitinib | 0.35 ± 0.05 μM (1.0) | 4.15 ± 0.39 μM (12.2)* | 68.3 ± 14.9 nM (0.9) | 298.5 ± 41.5 nM (4.1)* |
| Cisplatin | 11.45 ± 2.49 μM (1) | 12.11 ± 1.38 μM (1.1) | 2.10 ± 0.48 μM (1) | 1.88 ± 0.25 μM (0.9) |
| + 3 μM pelitinib | 10.16 ± 2.06 μM (0.9) | 11.72 ± 1.94 μM (1.0) | 1.95 ± 0.46 μM (0.9) | 1.98 ± 0.32 μM (0.9) |
Paclitaxel is a probe substrate anticancer drug for ABCB1 (Pgp).
Doxorubicin is a probe substrate anticancer drug for ABCC1 (MRP1).
Topotecan is a probe substrate anticancer drug for ABCG2.
Cisplatin is known to be a non-substrate for ABCB1, ABCC1 and ABCG2.
P < 0.05, difference from the conventional anticancer drug alone.
Inhibition kinetics of ABCB1- and ABCG2-mediated topotecan efflux by pelitinib
To further elucidate the mode of interaction between pelitinib and ABCB1/ABCG2, the efflux of the fluorescent substrate of both transporters (topotecan) at four different concentrations was studied in the absence and presence of pelitinib. Topotecan is a good ABCG2 substrate (Maliepaard et al., 1999) and a relative weaker ABCB1 substrate (Hendricks et al., 1992). As demonstrated in the Lineweaver–Burk plot (Figure 4), all curves intersect at the y-axis for both ABCB1 and ABCG2, suggesting a competitive inhibition. The Ki values was found to be 1.27 μM (ABCG2) and 5.49 μM (ABCB1), indicating a fairly strong interaction between pelitinib and the two transporters.
Figure 4.
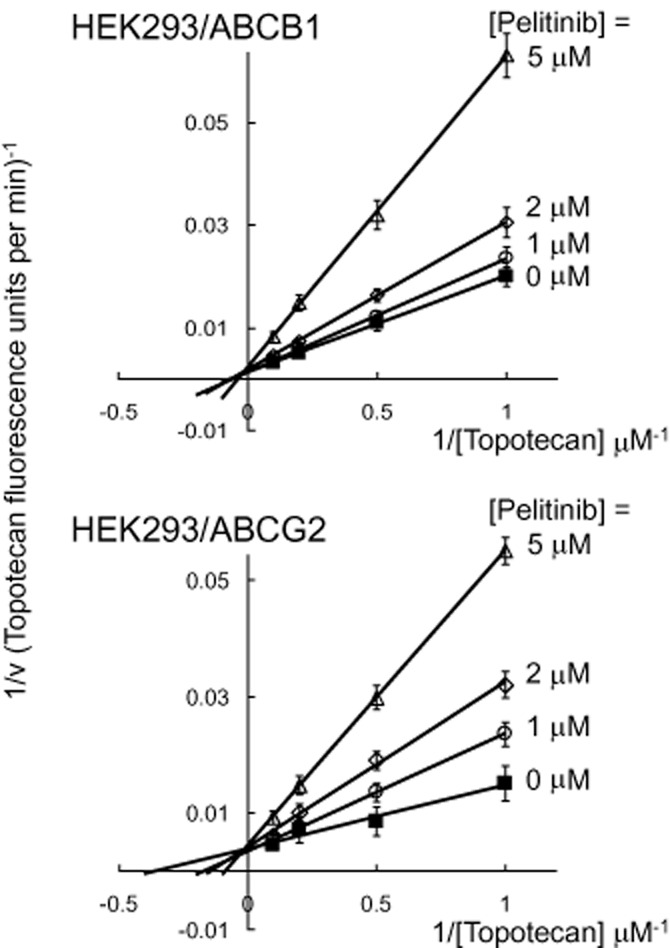
Inhibition kinetics of ABCB1- and ABCG2-mediated topotecan efflux by pelitinib. HEK293/ABCB1 or HEK293/ABCG2 cells were incubated with different concentrations of topotecan (1, 2, 5 and 10 μM) in the presence of four concentrations of pelitinib (0, 1, 2, or 5 μM) for 3 h. After a brief wash, the cells were incubated in topotecan-free medium continuing with or without pelitinib incubation to allow for efflux. The quantity of topotecan efflux was measured for 10 min by flow cytometry, which was calculated by subtracting the fluorescence signal obtained at 37°C from that at 0°C. The reciprocal of topotecan efflux rate is plotted against the reciprocal of pelitinib concentration using the Lineweaver–Burk plot. Because the lines converge on the y-axis, pelitinib is probably a competitive inhibitor of both ABCB1 and ABCG2 for the transport of topotecan. Each data point is presented as the mean ± SD from three independent experiments.
Modulation of ATPase activity of ABCB1 and ABCG2 by pelitinib
Drug transport activity of ABCB1 and ABCG2 are associated with ATP hydrolysis, which is modulated in the presence of their substrates or inhibitors. To understand further the mechanism of ABCB1 and ABCG2 inhibition by pelitinib, ABCB1- or ABCG2-mediated ATP hydrolysis in the presence of a range of different concentrations of pelitinib was measured. Pelitinib was found to stimulate the ATPase activity of both ABCB1 and ABCG2 in a concentration-dependent manner (Figure 5; *P < 0.01, **P < 0.05). ABCB1 ATPase activity was stimulated by pelitinib until it reached a plateau close to 60 nmol Pi·min−1·mg protein−1 and it remained steady at pelitinib concentration > 5 μM. On the other hand, a maximum ABCG2 ATPase activity of 74.6 ± 3.4 nmol Pi·min−1·mg protein−1 was attained in the presence of 1.25 μM pelitinib. At higher concentrations of pelitinib, a drop in the stimulated ABCG2 ATPase activity was observed.
Figure 5.
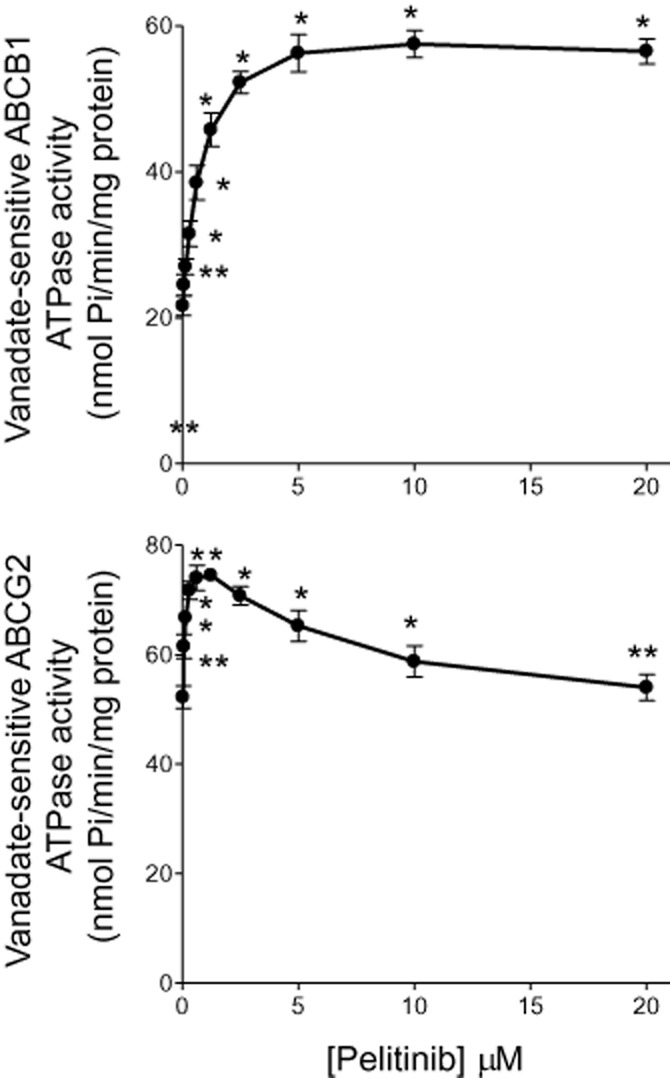
Effect of pelitinib on the ATPase activity of ABCB1 (top panel) and ABCG2 (bottom panel). The vanadate-sensitive ATPase activity of ABCB1 or ABCG2, in membrane protein obtained from the respective transporter-overexpressing High-Five insect cells, was determined at different concentrations of pelitinib. ATP hydrolysis was monitored by measuring the amount of inorganic phosphate released using a colorimetric assay. *P < 0.05, **P < 0.01, compared with the basal ATPase level in the absence of pelitinib.
Pelitinib did not alter the expression of ABCB1 and ABCG2 at both mRNA and protein levels
The reversal of ABCB1- and ABCG2-mediated drug resistance by pelitinib may also be associated with alteration of the transporter expression. Therefore, the mRNA and protein expressions of ABCB1 and ABCG2 were examined in A549 cells after incubating the cells with pelitinib at concentrations up to 3 μM for 48 h. Higher drug concentrations were not tested because prominent cell death was observed. At the tested concentrations, pelitinib did not affect the mRNA, cell surface staining (Figure 6) and total protein expressions of ABCB1 and ABCG2 (Figure 2B). Similar observations were also obtained in another NSCLC cell line H1975 (Supporting Information Fig. S2B and S3).
Figure 6.
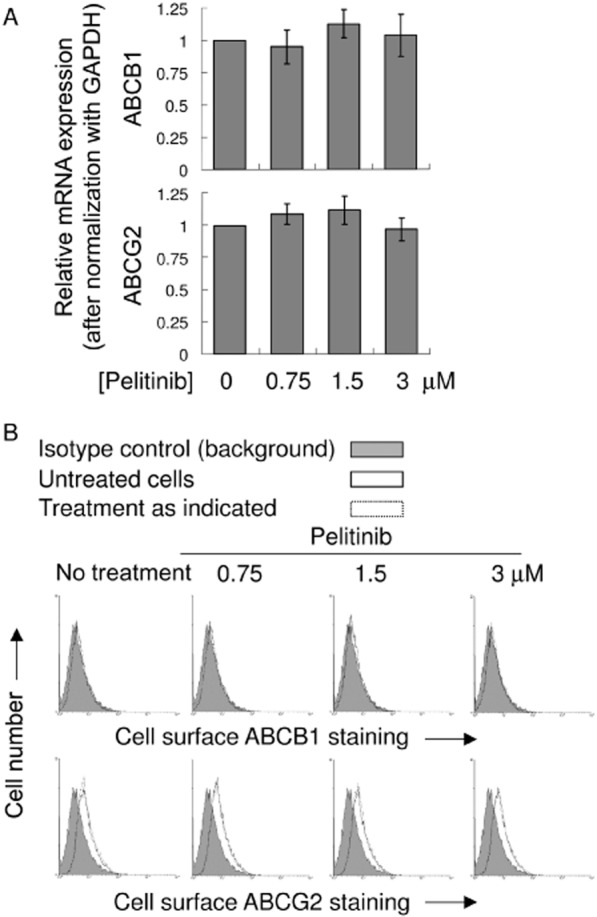
mRNA and cell surface expression of ABCB1 and ABCG2 in A549 after treatment with pelitinib. (A) PCR analysis in A549 cells treated with the indicated concentration of pelitinib for 48 h. mRNA expression was normalized with GAPDH. ABCB1/ABCG2 mRNA levels were expressed relative to that in the untreated A549 cells. (B) Representative histograms showing the cell surface staining of ABCB1 and ABCG2. Cells were trypsinized and incubated for 30 min in PE-labelled negative control antibody (shaded histogram) or UIC2/5D3 antibody (solid line, untreated cells; dashed line, pelitinib-treated cells) and analysed in a FACSsort flow cytometry. The distance between the UIC2/5D3 histogram (solid or dashed lines representing untreated and pretreated cells, respectively) and the shaded negative control antibody histogram provide an indication of the amount of ABCB1/ABCG2 protein expressed on the cell surface. The assays were repeated in three independent experiments.
Pelitinib specifically promoted apoptosis in combination with topotecan after hyperthermia exposure
A549 cells were treated with a combination of topotecan (20 nM) and low concentration of pelitinib (3 μM) for 48 h, after which the extent of apoptosis was measured. Pelitinib was used at 3 μM because it was previously found to inhibit both ABCB1 and ABCG2 at this concentration (Figure 3). While pelitinib did not appreciably cause apoptosis at this low concentration, its combination with topotecan was found to dramatically increase the proportion of apoptotic cells (69.5 ± 2.5% for drug combination vs. 42.9 ± 1.4% for topotecan alone; P < 0.01) after hyperthermia exposure (Figure 7). A similar enhancement of apoptotic effect by combination of pelitinib and topotecan under hyperthermia was also observed in another NSCLC cell line H1975 (Supporting Information Fig. S4).
Figure 7.
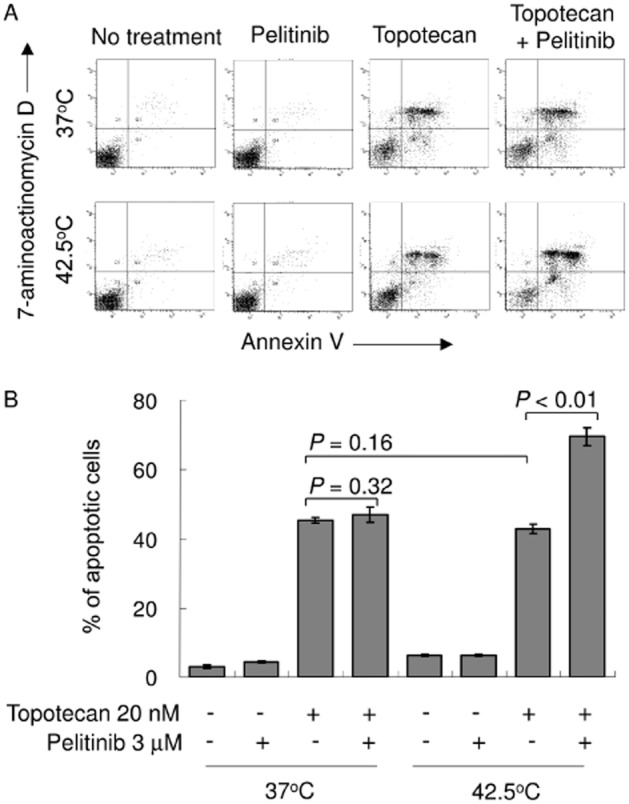
Pelitinib sensitized A549 cells to apoptosis specifically after exposure to hyperthermia. (A) A549 cells were exposed to topotecan alone (20 nM), pelitinib alone (3 μM) or their combination for 48 h before being harvested for the apoptosis assay. The cells were either exposed to hyperthermia at 42.5°C for 4 h or physiological temperature (37°C) before the drug treatments. A representative set of data from three independent experiments is shown. (B) Summary of apoptosis assay data from three independent experiments. Data are presented in histogram as means ± SD.
ABCB1- and ABCG2-overexpressing SP cells were targeted by pelitinib in combination with topotecan to commit apoptosis
We speculated that the ABCB1/ABCG2-overexpressing cell subpopulation was targeted by pelitinib in the combination treatment. Therefore, SP cells in A549 were sorted and examined after Hoechst 33342 staining. The SP subpopulation was defined as the diminished region in the presence of tariquidar, a potent dual inhibitor of both ABCB1 and ABCG2, which blocked the efflux of Hoechst 33342 dye. Interestingly, the proportion of SP cells in A549 was remarkably increased after hyperthermia exposure (42.5°C × 4 h) from 2.4 to 12.4% (Figure 8A). By transporter efflux assay, ABCG2 (and ABCB1, to a lesser extent) was found to be more highly expressed in SP cells than in non-SP cells (Figure 8B). Then, we investigated whether pelitinib had a selective chemosensitizing effect on the SP cells. As shown in Figure 8C, the SP cells were remarkably less susceptible to apoptosis upon topotecan treatment than non-SP cells, especially after hyperthermia exposure. Importantly, pelitinib was found to significantly enhance the sensitivity of SP cells to topotecan in a concentration-dependent manner and the effect was more pronounced in SP cells after hyperthermia treatment. In contrast, pelitinib did not exhibit appreciable effect on non-SP cells. A similar potentiation of apoptotic effect from combination of pelitinib and topotecan was also observed in SP sorted under hyperthermia in another NSCLC cell line H1975 (Supporting Information Fig. S5). As SP sorted by Hoechst dye exclusion may not truly represent the putative lung cancer stem cells, the experiment was also repeated by sorting out the CD133-overexpressing cells (a well-characterized lung cancer stem cell marker) and followed by the apoptosis assay. Interestingly, in A549, CD133-overexpressing cells were also increased after hyperthermia (Supporting Information Fig. S6A). More importantly, the combination of pelitinib and topotecan was found to preferentially potentiate the apoptotic effect in these CD133+ cells after hyperthermia (Supporting Information Fig. S6B).
Figure 8.
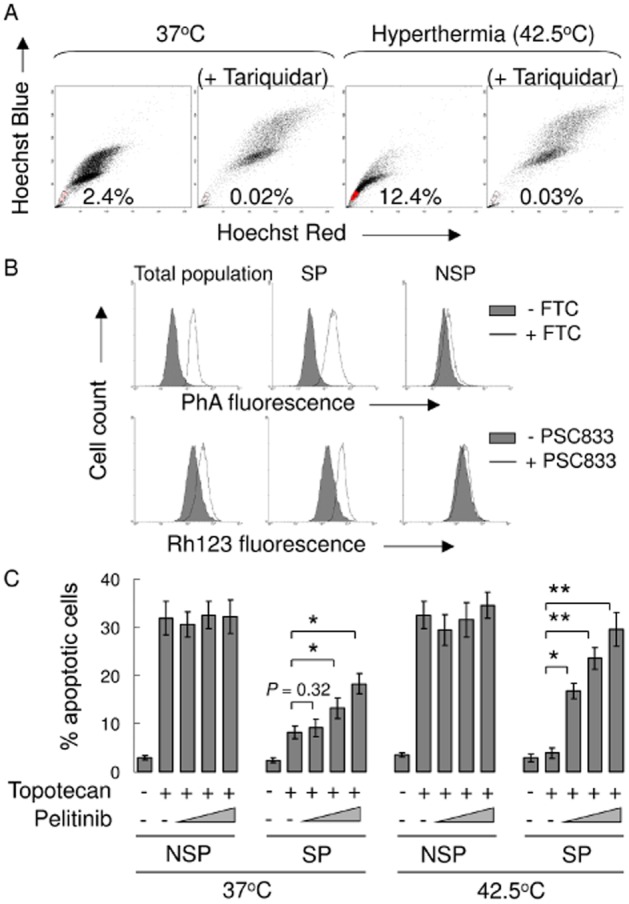
Pelitinib targeted the increased SP population exposed to hyperthermia and enhanced the apoptotic activity of topotecan. (A) A549 cells were stained with Hoechst 33342 as described in the Methods section. Gated on forward and side scatter to exclude debris, Hoechst red versus Hoechst blue was used to sort SP cells. (B) ABCB1 and ABCG2 efflux activity was assessed in total, SP and NSP cell populations. They were measured by comparing the retention of the respective fluorescent probe substrate for the two transporters (Rh123 for ABCB1 and PhA for ABCG2) in the presence (solid line) and absence (shaded histogram) of the specific inhibitors (PSC833 for ABCB1 and FTC for ABCG2). (C) SP and non-SP cells were treated with topotecan and pelitinib at the indicated concentrations for 48 h. Apoptosis was analysed by flow cytometry as the percentage of cells labelled by annexin V and 7-AAD. All of these experiments were repeated three times. Data from a representative experiment are shown. Columns, mean of triplicate measurements; *P < 0.05; **P < 0.005, compared with topotecan alone treatment in SP cells under the respective 37 or 42.5°C condition.
Pelitinib reduced the tumoursphere formation capacity of SP cells specifically after hyperthermia exposure
By expressing higher levels of ABCB1 and/or ABCG2, SP cells are more resistant to traditional chemotherapeutic drugs and may behave as CSCs, ultimately leading to cancer recurrence. In both A549 and H1975 cells, we investigated the tumoursphere formation capacity of SP and non-SP cells upon treatment with topotecan and its combination with pelitinib, with or without prior hyperthermia exposure. As expected, SP cells gave rise to more and larger tumourspheres and were more resistant to topotecan treatment at both 37 and 42.5°C, albeit more remarkable at the elevated temperature (Figure 9 and Supporting Information Fig. S7). Importantly, while pelitinib alone did not affect the tumoursphere formation capacity, the combination of pelitinib with topotecan was found to significantly reduce the number of tumourspheres formed and this effect was more pronounced after cell exposure to 42.5°C (Figure 9B and Supporting Information Fig. S7).
Figure 9.
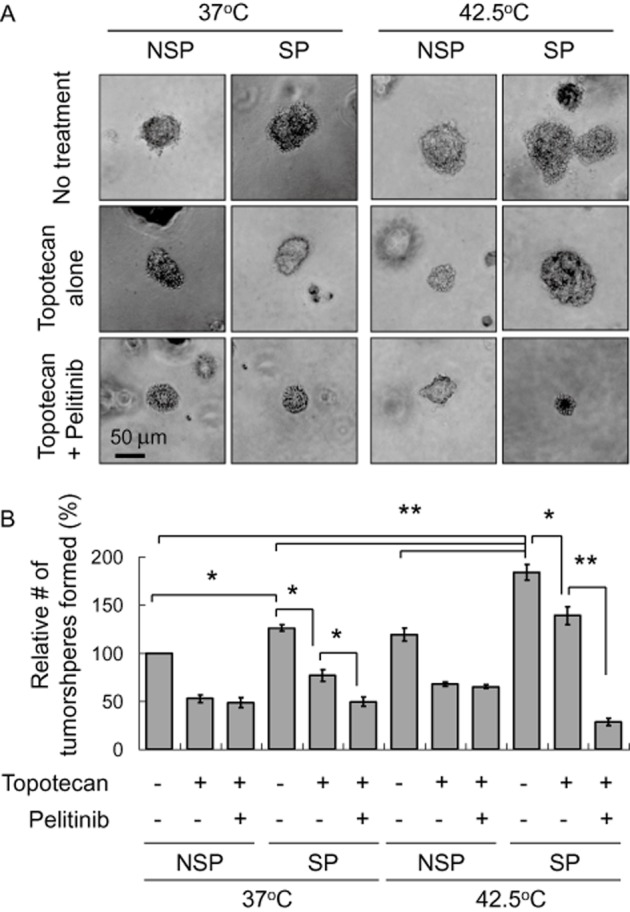
Tumoursphere formation assay of A549 cells treated with topotecan in the absence or presence of pelitinib at 37 or 42.5°C. Cells sorted after Hoechst staining were treated with topotecan alone or the combination of topotecan and pelitinib for 48 h. The cells (2 × 103·mL−1) were then cultured in serum-free DMEM medium with growth factors (10 ng·mL−1 EGF and bFGF each) for 12 days and the spheres were counted as indicated in the Methods section. (A) Representative images of spheres (magnification × 40). Scale bar, 50 μm. (B) Relative number of tumoursphere formed under the various treatment conditions. *P < 0.005; **P < 0.001.
Discussion and conclusions
This study sought to exploit the up-regulation of ABCB1/ABCG2 after hyperthermia and utilize the combination of pelitinib with other conventional anticancer drugs to specifically eradicate cancer cells. The most extensively studied MDR transporter, ABCB1/P-gp, is known to be inducible by heat and drugs (Miyazaki et al., 1992; Stein et al., 2001). It is therefore logical to speculate that hyperthermia may limit the potency of simultaneously administered anticancer drugs by increasing the expression of the efflux transporters. In three NSCLC cell lines tested (A549, H460 and H1975), both ABCB1 and ABCG2 were up-regulated at mRNA and protein levels by hyperthermia (Figure 1A, 2A and B & Supporting Information Figs S1, S2A and B). Surprisingly, while the cellular accumulation of topotecan (substrate anticancer drugs of both ABCB1 and ABCG2) was found to decrease after hyperthermia treatment (Figure 2C and D and Supporting Information Fig. S2C), the cytotoxicity of both drugs was not compromised (Supporting Information Table S1). Hyperthermia has been reported to cause direct damage to DNA (Speit and Schutz, 2013) and enhance intracellular drug response to chemotherapeutic drugs (Alcala et al., 2010), which may have compensated for the decrease in drug accumulation.
While the induction of ABCB1 by heat shock is well established (Miyazaki et al., 1992; Stein et al., 2001), less is known about the hyperthermia-mediated up-regulation of ABCG2, another key ABC transporter mediating MDR. We therefore performed promoter analysis and identified a HSE within the proximal ABCG2 promoter, which is responsible for the heat-induced up-regulation of the transporter (Figure 1B and C).
We have been studying the use of molecularly targeted TKIs to inhibit ABC transporters in the circumvention of MDR. In this study, a newly developed EGFR TKI pelitinib was chosen to investigate its targeting of ABCB1 and ABCG2 up-regulation for chemosensitization in lung cancer after hyperthermia exposure. Pelitinib was first evaluated for possible MDR reversal in cancer cell line models with defined overexpression of the three major MDR transporters (ABCB1/P-gp, ABCC1 and ABCG2). While pelitinib at concentrations below 3 μM did not appreciably affect cell proliferation, it was found to significantly potentiate the anticancer activity of other transporter substrate anticancer drugs [paclitaxel (ABCB1), topotecan (ABCG2) and doxorubicin (ABCC1)] in the corresponding transporter-overexpressing cell lines (Table 2010). ABCC10 is another highly expressed ABC transporter in lung cancer mediating drug resistance and it shares high sequence similarity with ABCB1, ABCC1 and ABCG2. Because pelitinib was found to inhibit all three major ABC transporters, it is likely that pelitinib can also inhibit ABCC10 and help circumvent the transporter-mediated drug resistance. To this end, a few TKIs, including imatinib and nilotinib, have been reported to inhibit ABCC10 (Shen et al., 2009).
Consistent with the observed potentiation of cytotoxicity in drug combination studies, pelitinib was found to inhibit the efflux of Rh123, calcein AM and PhA (ABCB1-, ABCC1- and ABCG2- fluorescent probe substrate) in the corresponding transporter-overexpressing resistant cancer cells and stably transfected cell lines (Figure 3). As only ABCB1 and ABCG2 were found to be up-regulated after hyperthermia in the lung cancer cell lines tested, more detailed investigations into the transporter inhibition by pelitinib was only performed on these two transporters. Results from the transport kinetic study revealed that pelitinib is probably a competitive inhibitor of both ABCB1 and ABCG2 (Figure 4). The Ki value of pelitinib for its inhibition of topotecan transport by ABCG2 was found to be higher than that of ABCB1, suggesting a higher affinity of pelitinib to ABCG2 than to ABCB1.
The ATPase assay is another widely used biochemical assay for the study of MDR transporter–drug interactions (Ambudkar, 1998). ABC transporters use the energy generated from ATP hydrolysis by ATPase to effectively transport their substrate drugs. Most TKIs [including apatinib (Mi et al., 2010), erlotinib (Shi et al., 2007) and lapatinib (Dai et al., 2008)] have been reported to modulate MDR transporters by stimulating the ATPase activity. The stimulation of ATPase activity of both ABCB1 and ABCG2 by pelitinib is consistent with the results from the transporter inhibition kinetic study, which suggest the TKI is a substrate of both transporters. In fact, a recent report by Hegedus et al also found that pelitinib is likely an ABCG2 substrate and that the TKI may be useful for enhancing the cytotoxicity of ABCG2-substrate anticancer drugs (Hegedus et al., 2012). However, to the best of our knowledge, this study is the first report demonstrating the inhibition of multiple MDR transporters (i.e. ABCB1, ABCC1 and ABCG2) and the subsequent circumvention of transporter-mediated drug resistance by pelitinib.
The effect of pelitinib on ABCB1 and ABCG2 expression was also evaluated. At the transporter-inhibitory and resistance-reversal concentrations, pelitinib did not affect the expression of either transporter in the lung cancer cell line A549 (Figure 6), H1975 (Supporting Information Fig. S3) and a normal colon epithelial cell line CCD841 CoN (data not shown). This is beneficial because of a lower chance of pharmacokinetic drug-drug interactions, although a more in-depth investigation in vivo is warranted.
The potentiation of the anticancer activity of topotecan (substrate anticancer drug of both ABCB1 and ABCG2) by pelitinib was demonstrated by the apoptosis assay. Importantly, a significant increase in apoptosis was only observed for the combination of topotecan and pelitinib after hyperthermia exposure (Figure 7 – A549 cells; Supporting Information Fig. S4 – H1975 cells). The likely explanation is that pelitinib specifically inhibits the up-regulated ABCB1 and ABCG2 after hyperthermia to elicit a potentiation of apoptosis. It is noted that A549 cells express wild-type EGFRs, whereas H1975 cells express the secondary EGFR T790M mutation. Therefore, the potentiation of apoptosis by the drug combination involving pelitinib is not specific to the status of the EGFR. To further confirm the specific targeting of cancer cells with up-regulated levels of the two transporters by pelitinib after hyperthermia, SP cells were sorted out after Hoechst staining and evaluated for the extent of apoptosis after a combined treatment with topotecan and pelitinib (Figure 8 – A549 cells; Supporting Information Fig. S5 – H1975 cells). The SP cells represent the cell subpopulation overexpressing ABCB1/ABCG2 (Figure 8B) and it was in fact increased after hyperthermia (12.4% at 42.5°C vs. 2.4% at 37°C). Importantly, while the SP cells isolated (with or without prior hyperthermia exposure) were more resistant to topotecan-induced apoptosis, the combination of pelitinib with topotecan was found to specifically enhance the apoptosis in SP cells sorted after hyperthermia treatment (Figure 8C – A549 cells; Supporting Information Fig. S5C – H1975 cells). As SP cells sorted by Hoechst dye exclusion may not reliably represent the putative cancer stem cells, the potentiation of apoptosis by the combination of pelitinib with topotecan was also performed and confirmed in the CD133-overexpressing cell population after hyperthermia (Supporting Information Fig. S6). It is also noteworthy that the concentration of pelitinib (with the low end at 1 μM) effective at potentiating apoptosis in drug combination is achievable in vivo (Erlichman et al., 2006), therefore highlighting the clinical relevance of our findings.
Tumoursphere formation capacity is an important parameter to define the more malignant and resistant cancer cells probably responsible for cancer recurrence. As expected, SP cells sorted from both A549 and H1975 formed more tumourspheres in suspension culture and were less responsive to topotecan treatment. Importantly, the combination of pelitinib and topotecan were found to specifically and dramatically reduce the number of tumourspheres formed from SP cells after hyperthermia treatment (Figure 9B – A549 cells; Supporting Information Fig. S7B – H1975 cells), suggesting its usefulness at helping to eradicate the most resistant cancer cells from the whole cell population.
In conclusion, the combination of pelitinib and other conventional anticancer drugs with concomitant hyperthermia exposure may be adopted as a novel means to eradicate the CSCs responsible for cancer recurrence. Further mechanistic investigations and animal studies are warranted to fully understand and optimize the beneficial drug combinations.
Acknowledgments
This work was supported by the National Natural Science Foundation of China/Research Grants Council of Hong Kong Joint Research Scheme 2010/2011 (Project No. N_CUHK443/10) and the CUHK Direct Grant for Research (4054035) to K. K. W. T. We would like to thank Dr Susan Bates (National Cancer Institute, NIH) and Professor Robert Clarke (Georgetown University, USA) for the MDR transporters-overexpressing cancer cell lines and the various specific transporter inhibitors.
Glossary
- ABC
ATP-binding cassette
- CSC
cancer stem-like cell
- FTC
fumitremorgin C
- HSE
heat shock element
- MDR
multidrug resistance
- NSP
non-SP population
- PhA
pheophorbide A
- Rh123
rhodamine 123
- SP
side population
Author contributions
K. K. W. T., D. C. P., Y. W. and F. W. performed the research. K. K. W. T., G. L. and L. F. designed the research study. L. F. contributed essential reagents. K. K. W. T. analysed the data. K. K. W. T. and L. F. wrote the paper.
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Real-time PCR analysis of ABCB1, ABCC1 and ABCG2 mRNA expression in A549, H1975 or H460 NSCLC cell lines. Relative ABCB1, ABCC1 or ABCG2 expression is shown after normalization with GAPDH. *P < 0.05, compared with cell culture at 37°C. (A) Temperature-dependent up-regulation of ABCB1 and ABCG2 in A549 cells. Cells were incubated at physiological temperature 37°C or elevated temperature (39, 41 or 42.5°C) for 4 h before being harvested for PCR analysis. (B) Up-regulation of ABCB1 and ABCG2 mRNA level by hyperthermia (42.5°C) in other NSCLC cell lines (H460 & H1975). *P < 0.05, compared with cell culture at 37°C.
Figure S2 Cell surface expression and functional analysis of ABCG2 and ABCB1 in H1975 cells (harbouring EGF receptor T790M secondary mutation) with or without hyperthermia (42.5°C) treatment. (A) Cell surface ABCG2 or ABCB1 staining of H1975 cells with or without hyperthermia (42.5°C); pretreatment was quantified by subtracting the fluorescence signal from 5D3 or UIC2 labelling by that from the control IgG isotype antibody labelling. Mean ± SD from three independent experiments is shown. *P < 0.05, compared with cell culture at 37°C. (B) Western blot analysis of total ABCB1 or ABCG2 protein expression in H1975 cells with or without 4 h hyperthermia (42.5°C) treatment. It is also noted that pelitinib treatment did not alter the up-regulated ABCB1 or ABCG2 protein expression after hyperthermia (42.5°C) treatment. (C) Decreased cellular accumulation of topotecan in H1975 cells after hyperthermia as detected by flow cytometry. Drug incubation and hyperthermia treatment were the same as in (Figure 1D). After the drug incubation, the cells were collected, washed twice in ice-cold PBS and retention of the fluorescence inside the cells was analysed by flow cytometry. Flow cytometry histogram from a representative experiment is shown.
Figure S3 mRNA and cell surface expression of ABCB1 and ABCG2 in H1975 cells (harbouring EGF receptor T790M secondary mutation) after treatment with pelitinib. (A) PCR analysis in H1975 cells treated with the indicated concentration of pelitinib for 48 h. mRNA expression was normalized with GAPDH. ABCB1/ABCG2 mRNA levels were expressed relative to that in the untreated H1975 cells. (B) Representative histograms showing the cell surface staining of ABCB1 and ABCG2. Cells were trypsinized and incubated for 30 min in PE-labelled negative control antibody (shaded histogram) or UIC2/5D3 antibody (solid line, untreated cells; dashed line, pelitinib-treated cells) and analysed in a FACSsort flow cytometry. The distance between the UIC2/5D3 histogram (solid or dashed lines representing untreated and pretreated cells, respectively) and the shaded negative control antibody histogram provide an indication of the amount of ABCB1/ABCG2 protein expressed on the cell surface. The assays were repeated in three independent experiments.
Figure S4 Pelitinib sensitized H1975 cells (harbouring the secondary EGF receptor T790M mutation) to apoptosis specifically after exposure to hyperthermia. H1975 cells were allowed to expose to topotecan alone (20 nM), pelitinib alone (3 μM) or their combination for 48 h before harvest for apoptosis assay. The cells were either exposed to hyperthermia at 42.5°C for 4 h or physiological temperature (37°C) before the drug treatments. Summary of apoptosis assay data from three independent experiments is shown. Data are presented in histogram as means ± SD.
Figure S5 Pelitinib targeted the increased side population (SP) in H1975 cells (harbouring the secondary EGF receptor T790M mutation) under hyperthermia and enhanced the apoptotic activity of topotecan. (A) H1975 cells were stained with Hoechst 33342 as described in the Methods section. Gated on forward and side scatter to exclude debris, Hoechst red versus Hoechst blue was used to sort SP cells. (B) ABCB1 and ABCG2 efflux activity was assessed in total, SP and NSP cell population. They were measured by comparing the retention of the respective fluorescent probe substrate for the two transporters (Rh123 for ABCB1 and PhA for ABCG2) in the presence (solid line) and absence (shaded histogram) of the specific inhibitor (PSC833 for ABCB1 and FTC for ABCG2). (C) Sorted SP and non-SP cells treated with topotecan and pelitinib at the indicated concentrations for 48 h. Apoptosis was analysed by flow cytometry as the percentage of cells labelled by annexin V and 7-AAD. All of these experiments were repeated three times. Data from a representative experiment is shown. Columns, mean of triplicate measurements; *P < 0.05; **P < 0.005, compared with topotecan alone treatment in SP cells under the respective 37 or 42.5°C condition.
Figure S6 Pelitinib also targeted the increased CD133+ population in A549 cells under hyperthermia and enhanced the apoptotic activity of topotecan. (A) H1975 cells were labelled with CD133 antibody and sorted out by FACS technique. (B) Sorted CD133+ and CD133− cells treated with topotecan and pelitinib at the indicated concentrations for 48 h. Apoptosis was analysed by flow cytometry as the percentage of cells labelled by annexin V and 7-AAD. All of these experiments were repeated three times. Data from a representative experiment is shown. Columns, mean of triplicate measurements; *P < 0.05; **P < 0.005, compared with topotecan alone treatment in SP cells under the respective 37 or 42.5°C condition.
Figure S7 Tumoursphere formation assay of H1975 cells (harbouring the secondary EGF receptor T790M mutation) treated with topotecan in the absence or presence of pelitinib at 37 or 42.5°C. Cells sorted after Hoechst staining were treated with topotecan alone or the combination of topotecan and pelitinib for 48 h. The cells (2 × 103·mL−1) were then cultured in serum-free DMEM medium with growth factors (10 ng·mL−1 EGF and bFGF each) for 12 days and counted the spheres as indicated in the Methods section. (A) Representative images of spheres (magnification × 40). Scale bar, 50 μm. (B) Relative number of tumoursphere formed under the various treatment conditions. *P < 0.005; **P < 0.001.
Table S1 Anticancer activity of topotecan and etoposide in A549 and H460 human non-small cell lung cancer cells, with or without prior hyperthermia exposure (42.5°C × 4 h).
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala MA, Jr, Park K, Yoo J, Lee DH, Park BH, Lee BC, et al. Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway and growth of xenograft tumors. J Cell Biochem. 2010;110:1073–1081. doi: 10.1002/jcb.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: catalytic receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontet C, Bodin F, Michal Y. Potential interactions between antitubulin agents and temperature: implications for modulation of multidrug resistance. Clin Cancer Res. 1998;4:1563–1566. [PubMed] [Google Scholar]
- Erlichman C, Hidalgo M, Boni JP, Martins P, Quinn SE, Zacharchuk C, et al. Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2006;24:2252–2260. doi: 10.1200/JCO.2005.01.8960. [DOI] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- Hegedus C, Truta-Feles K, Antalffy G, Varady G, Nemet K, Ozvegy-Laczka C, et al. Interaction of the EGFR inhibitors gefitinib, vandetanib, pelitinib and neratinib with ABCG2 multidrug transporter: implications for the emergence and reversal of cancer drug resistance. Biochem Pharmacol. 2012;84:260–267. doi: 10.1016/j.bcp.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Hendricks CB, Rowinsky EK, Grochow LB, Donehower RC, Kaufmann SH. Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue. Cancer Res. 1992;52:2268–2278. [PubMed] [Google Scholar]
- Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonessa F, Green D, Licht T, Wright A, Wingate-Legette K, Lippman J, et al. MDA435/LCC6 and MDA435/LCC6MDR1: ascites models of human breast cancer. Br J Cancer. 1996;73:154–161. doi: 10.1038/bjc.1996.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia. 2003;19:252–266. doi: 10.1080/0265673031000065042. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells. Demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- Miyazaki M, Kohno K, Uchiumi T, Tanimura H, Matsuo K, Nasu M, et al. Activation of human multidrug resistance-1 gene promoter in response to heat shock. Biochem Biophys Res Commun. 1992;187:677–684. doi: 10.1016/0006-291x(92)91248-o. [DOI] [PubMed] [Google Scholar]
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–468. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
- O'Brien M, Eckardt J, Ramlau R. Recent advances with topotecan in the treatment of lung cancer. Oncologist. 2007;12:1194–1204. doi: 10.1634/theoncologist.12-10-1194. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangeli P, Mondovi B. On the biochemical basis of tumour damage by hyperthermia. In: Baronzio GF, Hager ED, editors. Hyperthermia in Cancer Treatment: A Primer. Germany: Landes Bioscience Open Access Book Library; 2006. pp. 1–9. [Google Scholar]
- Schildkopf P, Ott OJ, Frey B, Wadepohl M, Sauer R, Fietkau R, et al. Biological rationales and clinical applications of temperature controlled hyperthermia – implications for multimodal cancer treatments. Curr Med Chem. 2010;17:3045–3057. doi: 10.2174/092986710791959774. [DOI] [PubMed] [Google Scholar]
- Schneider E, Horton JK, Yang CH, Nakagawa M, Cowan KH. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994;54:152–158. [PubMed] [Google Scholar]
- Shen T, Kuang YH, Ashbby CR, Lei Y, Chen A, Zhou Y, et al. Imatinib and nilotinib reverse multidrug resistance in cancer cells by inhibiting the efflux activity of the MRP7 (ABCC10) PLoS ONE. 2009;4:e7520. doi: 10.1371/journal.pone.0007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- Skehan P, Stornet R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Speit G, Schutz P. Hyperthermia – induced genotoxic effects in human A549 cells. Mutat Res. 2013;747–748:1–5. doi: 10.1016/j.mrfmmm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Stein U, Jurchott K, Walther W, Bergmann S, Schlag PM, Royer HD. Hyperthermia-inducible nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J Biol Chem. 2001;276:28562–28569. doi: 10.1074/jbc.M100311200. [DOI] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- To KK, Tomlinson B. Targeting the ABCG2-overexpressing multidrug resistant (MDR) cancer cells by PPARγ agonists. Br J Pharmacol. 2013;170:1137–1151. doi: 10.1111/bph.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Zhan Z, Bates SE. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol Cell Biol. 2006;26:8572–8585. doi: 10.1128/MCB.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Poon DC, Chen XG, Fu LW. Volasertib (BI 6727), a novel polo-like kinase inhibitor, reverses ABCB1 and ABCG2-mediated multidrug resistance in cancer cells. J Cancer Ther Res. 2013;2:13. [Google Scholar]
- Tong XZ, Wang F, Liang S, Zhang X, He JH, Chen XG, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83:586–597. doi: 10.1016/j.bcp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Wang F, Mi YJ, Chen XG, Wu XP, Liu Z, Chen SP, et al. Axitinib targeted cancer stemlike cells to enhance efficacy of chemotherapeutic drugs via inhibiting the drug transport function of ABCG2. Mol Med. 2012;18:887–898. doi: 10.2119/molmed.2011.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- Zheng LS, Wang F, Li YH, Zhang X, Chen LM, Liang YJ, et al. Vandetinib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport function. PLoS ONE. 2009;4:e5172. doi: 10.1371/journal.pone.0005172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WJ, Zhang X, Cheng C, Wang F, Wang XK, Liang YJ, et al. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br J Pharmacol. 2012;166:1669–1683. doi: 10.1111/j.1476-5381.2012.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwischenberger JB, Vertrees RA, Woodson LC, Bedell EA, Alpard SK, McQuitty CK, et al. Percutaneous venovenous perfusion-induced systemic hyperthermia for advanced non-small cell lung cancer: initial clinical experience. Ann Thorac Surg. 2001;72:234–242. doi: 10.1016/s0003-4975(01)02673-x. [DOI] [PubMed] [Google Scholar]
- Zwischenberger JB, Vertrees RA, Bedell EA, McQuitty CK, Chernin JM, Woodson LC. Percutaneous venovenous Perfusion-induced systemic hyperthermia for lung cancer: a phase I safety study. Ann Thorac Surg. 2004;77:1916–1925. doi: 10.1016/j.athoracsur.2003.10.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Real-time PCR analysis of ABCB1, ABCC1 and ABCG2 mRNA expression in A549, H1975 or H460 NSCLC cell lines. Relative ABCB1, ABCC1 or ABCG2 expression is shown after normalization with GAPDH. *P < 0.05, compared with cell culture at 37°C. (A) Temperature-dependent up-regulation of ABCB1 and ABCG2 in A549 cells. Cells were incubated at physiological temperature 37°C or elevated temperature (39, 41 or 42.5°C) for 4 h before being harvested for PCR analysis. (B) Up-regulation of ABCB1 and ABCG2 mRNA level by hyperthermia (42.5°C) in other NSCLC cell lines (H460 & H1975). *P < 0.05, compared with cell culture at 37°C.
Figure S2 Cell surface expression and functional analysis of ABCG2 and ABCB1 in H1975 cells (harbouring EGF receptor T790M secondary mutation) with or without hyperthermia (42.5°C) treatment. (A) Cell surface ABCG2 or ABCB1 staining of H1975 cells with or without hyperthermia (42.5°C); pretreatment was quantified by subtracting the fluorescence signal from 5D3 or UIC2 labelling by that from the control IgG isotype antibody labelling. Mean ± SD from three independent experiments is shown. *P < 0.05, compared with cell culture at 37°C. (B) Western blot analysis of total ABCB1 or ABCG2 protein expression in H1975 cells with or without 4 h hyperthermia (42.5°C) treatment. It is also noted that pelitinib treatment did not alter the up-regulated ABCB1 or ABCG2 protein expression after hyperthermia (42.5°C) treatment. (C) Decreased cellular accumulation of topotecan in H1975 cells after hyperthermia as detected by flow cytometry. Drug incubation and hyperthermia treatment were the same as in (Figure 1D). After the drug incubation, the cells were collected, washed twice in ice-cold PBS and retention of the fluorescence inside the cells was analysed by flow cytometry. Flow cytometry histogram from a representative experiment is shown.
Figure S3 mRNA and cell surface expression of ABCB1 and ABCG2 in H1975 cells (harbouring EGF receptor T790M secondary mutation) after treatment with pelitinib. (A) PCR analysis in H1975 cells treated with the indicated concentration of pelitinib for 48 h. mRNA expression was normalized with GAPDH. ABCB1/ABCG2 mRNA levels were expressed relative to that in the untreated H1975 cells. (B) Representative histograms showing the cell surface staining of ABCB1 and ABCG2. Cells were trypsinized and incubated for 30 min in PE-labelled negative control antibody (shaded histogram) or UIC2/5D3 antibody (solid line, untreated cells; dashed line, pelitinib-treated cells) and analysed in a FACSsort flow cytometry. The distance between the UIC2/5D3 histogram (solid or dashed lines representing untreated and pretreated cells, respectively) and the shaded negative control antibody histogram provide an indication of the amount of ABCB1/ABCG2 protein expressed on the cell surface. The assays were repeated in three independent experiments.
Figure S4 Pelitinib sensitized H1975 cells (harbouring the secondary EGF receptor T790M mutation) to apoptosis specifically after exposure to hyperthermia. H1975 cells were allowed to expose to topotecan alone (20 nM), pelitinib alone (3 μM) or their combination for 48 h before harvest for apoptosis assay. The cells were either exposed to hyperthermia at 42.5°C for 4 h or physiological temperature (37°C) before the drug treatments. Summary of apoptosis assay data from three independent experiments is shown. Data are presented in histogram as means ± SD.
Figure S5 Pelitinib targeted the increased side population (SP) in H1975 cells (harbouring the secondary EGF receptor T790M mutation) under hyperthermia and enhanced the apoptotic activity of topotecan. (A) H1975 cells were stained with Hoechst 33342 as described in the Methods section. Gated on forward and side scatter to exclude debris, Hoechst red versus Hoechst blue was used to sort SP cells. (B) ABCB1 and ABCG2 efflux activity was assessed in total, SP and NSP cell population. They were measured by comparing the retention of the respective fluorescent probe substrate for the two transporters (Rh123 for ABCB1 and PhA for ABCG2) in the presence (solid line) and absence (shaded histogram) of the specific inhibitor (PSC833 for ABCB1 and FTC for ABCG2). (C) Sorted SP and non-SP cells treated with topotecan and pelitinib at the indicated concentrations for 48 h. Apoptosis was analysed by flow cytometry as the percentage of cells labelled by annexin V and 7-AAD. All of these experiments were repeated three times. Data from a representative experiment is shown. Columns, mean of triplicate measurements; *P < 0.05; **P < 0.005, compared with topotecan alone treatment in SP cells under the respective 37 or 42.5°C condition.
Figure S6 Pelitinib also targeted the increased CD133+ population in A549 cells under hyperthermia and enhanced the apoptotic activity of topotecan. (A) H1975 cells were labelled with CD133 antibody and sorted out by FACS technique. (B) Sorted CD133+ and CD133− cells treated with topotecan and pelitinib at the indicated concentrations for 48 h. Apoptosis was analysed by flow cytometry as the percentage of cells labelled by annexin V and 7-AAD. All of these experiments were repeated three times. Data from a representative experiment is shown. Columns, mean of triplicate measurements; *P < 0.05; **P < 0.005, compared with topotecan alone treatment in SP cells under the respective 37 or 42.5°C condition.
Figure S7 Tumoursphere formation assay of H1975 cells (harbouring the secondary EGF receptor T790M mutation) treated with topotecan in the absence or presence of pelitinib at 37 or 42.5°C. Cells sorted after Hoechst staining were treated with topotecan alone or the combination of topotecan and pelitinib for 48 h. The cells (2 × 103·mL−1) were then cultured in serum-free DMEM medium with growth factors (10 ng·mL−1 EGF and bFGF each) for 12 days and counted the spheres as indicated in the Methods section. (A) Representative images of spheres (magnification × 40). Scale bar, 50 μm. (B) Relative number of tumoursphere formed under the various treatment conditions. *P < 0.005; **P < 0.001.
Table S1 Anticancer activity of topotecan and etoposide in A549 and H460 human non-small cell lung cancer cells, with or without prior hyperthermia exposure (42.5°C × 4 h).


