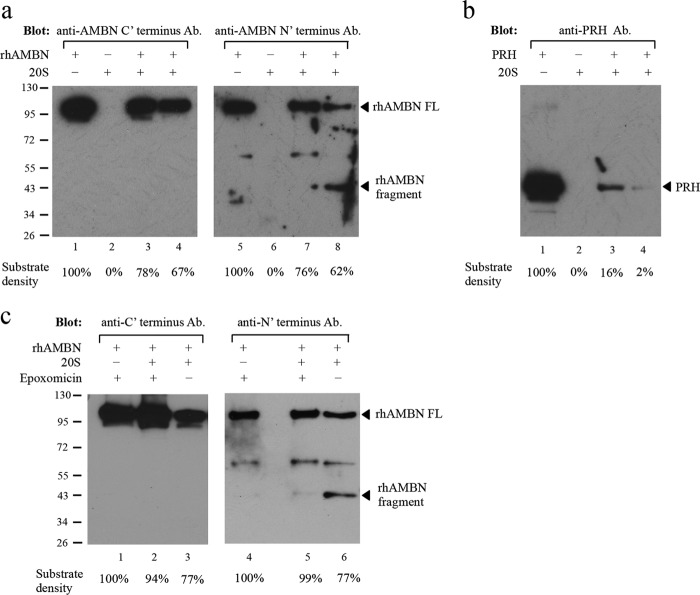
FIGURE 6.
Digestion of ameloblastin with 20S proteasome in vitro. a, Western blot with the ameloblastin C-terminal antibody (ab116347, Abcam) (lanes 1–4) or with the N-terminal antibody (N-18, Santa Cruz Biotechnology) (lanes 5–8). Lanes 1 and 5, recombinant human ameloblastin (rhAMBN) alone in assay buffer (50 mm Tris buffer (pH 7.0)) and the band at ∼100 kDa corresponds to the intact rhAMBN protein; lanes 2 and 6, purified 20S proteasome alone in assay buffer and no specific band was detected by either of the ameloblastin antibodies; lanes 3 and 7, rhAMBN incubated with purified 20S proteasome in assay buffer at 37 °C for 1 h with decreased intensity of the full-length rhAMBN band (∼100 kDa); lanes 4 and 8, rhAMBN incubated with purified 20S proteasome in assay buffer at 37 °C for 3 h, and the intensity of the full-length rhAMBN band (∼100 kDa) diminished further. Furthermore, with 20S digestion, a band corresponding to a cleavage product (∼43 kDa) was detected only by the N-terminal antibody (lane 8). Equal amount of rhAMBN was used in each lane. b, positive control of recombinant PRH demonstrated that the purified 20S proteasome used in the digestion assay retained its proteolytic activity. Equal amount of PRH was used in each lane. c, inhibition of proteasome digestion of ameloblastin by epoxomicin. Western blot with ameloblastin antibody specific to the C terminus (ab116347) (lanes 1–3) or the N terminus (N-18) (lanes 4–6) was used to detect ameloblastin with or without 50 μm epoxomicin. In the presence of epoxomicin and the 20S proteasome, the quantity of ameloblastin protein was not reduced dramatically (lanes 2 and 5). In contrast, without epoxomicin, incubation of rhAMBN with 20S resulted in diminution of ameloblastin protein quantity (lanes 3 and 6) with the release of a smaller molecular weight fragment (lane 6). Equal amounts of rhAMBN were used in each lane. Densitometry tracing of 20S proteasome digestion for rhAMBN and PRH is shown as a percentage of the total substrate mass used in each lane.