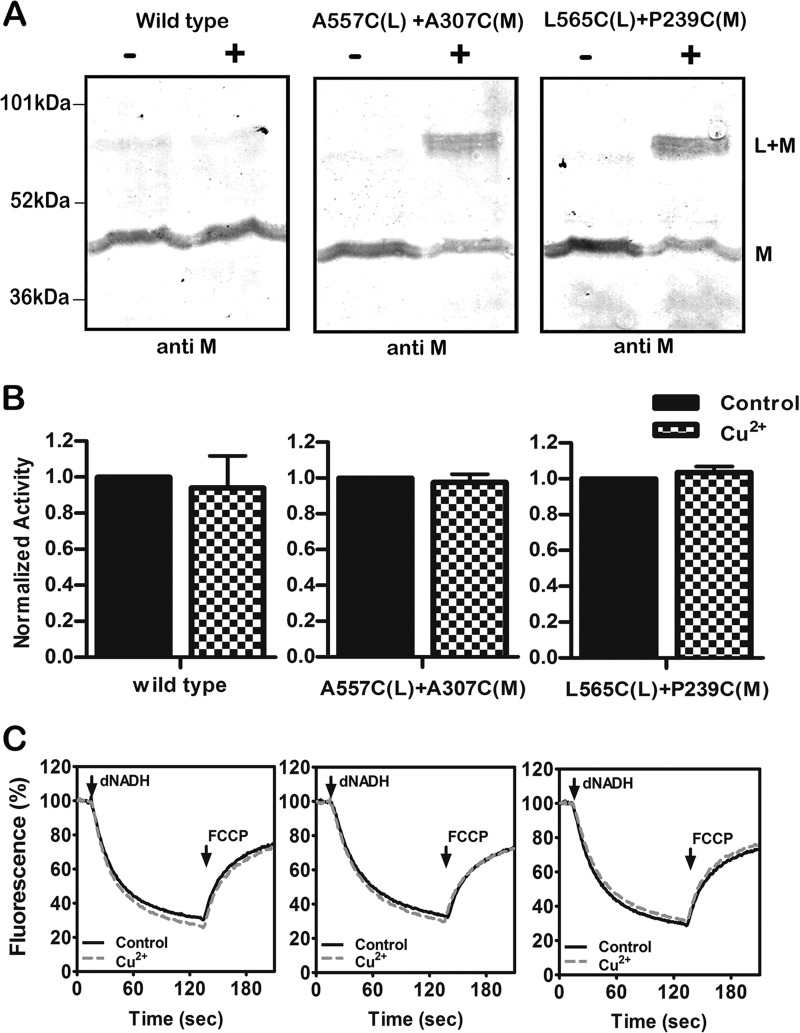
FIGURE 3.
Cross-linking between the HL helix and subunit M using Cu2+ ions. A, representative immunoblots of membrane samples from the wild type strain and from two mutants, A557C (nuoL) + A307C (nuoM) and L565C (nuoL) + P239C (nuoM), are shown with and without treatment with Cu2+ ions to promote disulfide formation. The antibody used was against subunit M. B, the effect of Cu2+ treatment on the deamino-NADH oxidase activity of the wild type strain and the same two mutants is shown. Results are the means and S.E. of at least four measurements from at least two membrane preparations. C, proton translocation assays of the wild type and the same two mutants with and without treatment with Cu2+ ions. The fluorescence quenching of ACMA was initiated by deamino-NADH and was reversed by the addition of FCCP.