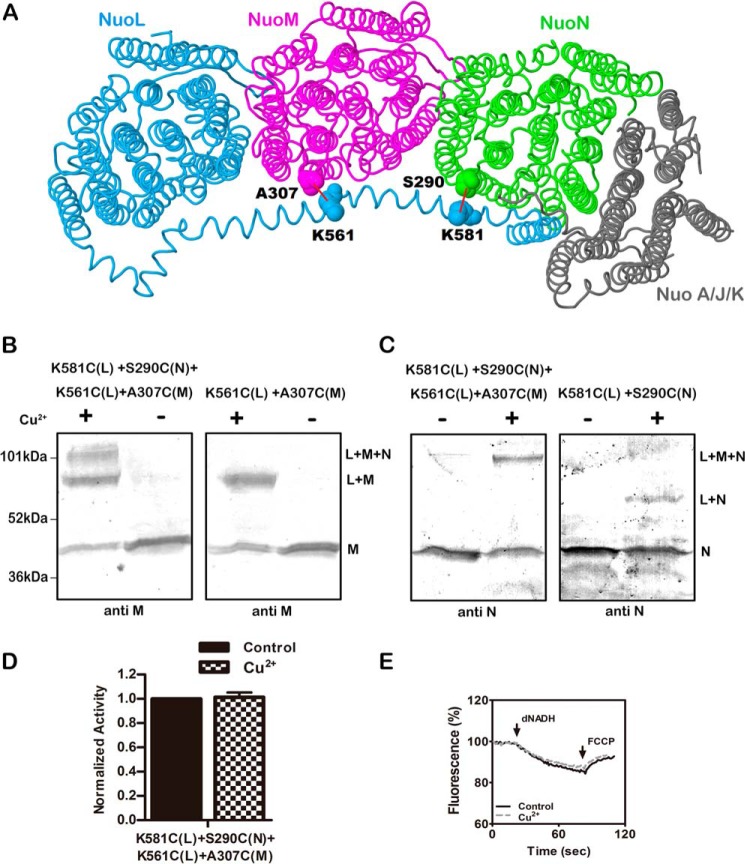
FIGURE 6.
Double cross-linking between the HL helix and both subunits M and N. A, schematic view of the membrane arm of Complex I showing the sites of the two cross-links. Subunit L is colored blue, subunit M is magenta, subunit N is green, and subunits A, J, and K are colored gray. Lys-561 and Lys-581 are in the HL of subunit L. Ala-307 is in subunit M, and Ser-290 is in subunit N. Both of these residues are from the cytoplasmic end of TM9 near the short loop that connects to TM10. The image was developed from Protein Data Bank code 3rko (16). B and C, representative immunoblots of membrane samples from K561C (nuoL) + K581C (nuoL) + A307C (nuoM) + S290C (nuoN) are shown with and without treatment Cu2+ ions to promote cross-link formation. At the left (B), the results are compared with the double mutant K561C (nuoL) + A307C (nuoM). Both blots were probed with antibody to subunit M. At the right (C), the results are compared with the double mutant K581C (nuoL) + S290C (nuoN). Both blots were probed with antibody to subunit N. D, the effect of Cu2+ ion treatment on the deamino-NADH oxidase activity of the quadruple mutant is shown. Results are the means and S.E. of at least four measurements from at least two membrane preparations. E, proton translocation assays of the quadruple mutant with and without treatment with Cu2+ ions are shown. The fluorescence quenching of ACMA was initiated by deamino-NADH and was reversed by the addition of FCCP.