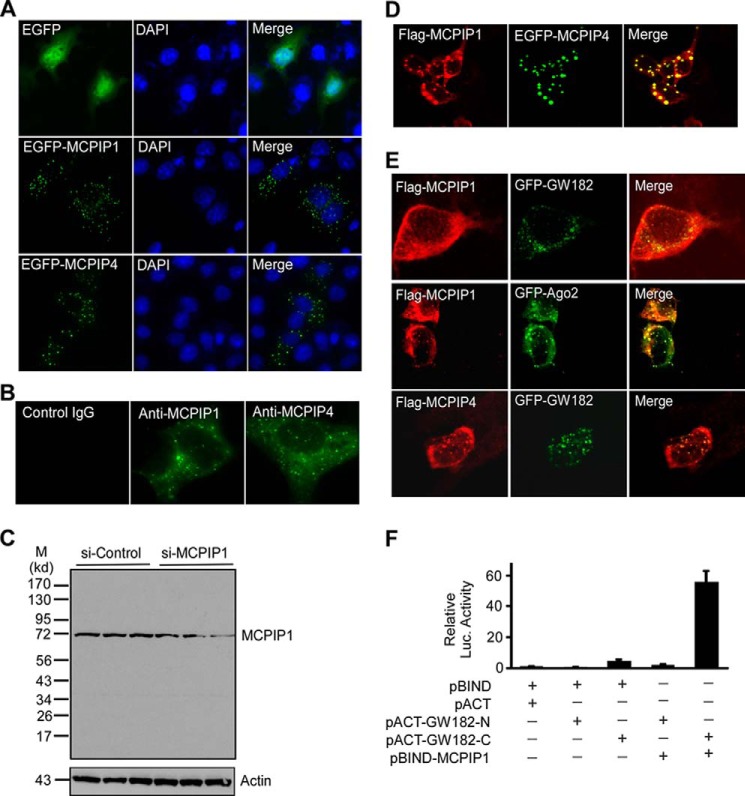
FIGURE 2.
MCPIP1 and MCPIP4 are co-localized in GW-body. A, COS-7 cells were transfected with the plasmids encoding EGFP or EGFP-MCPIP1 or EGFP-MCPIP4. The cells were visualized by confocal microscopy. The nuclei were stained by DAPI. B, RAW264.7 cells were stimulated with Pam3CSK4 for 6 h and then fixed and incubated with the primary ant-MCPIP1 or anti-MCPIP4 or control IgG. Then cells were visualized by Alexa Fluor488-labeled second antibody, and images were taken by confocal microscopy. C, RAW264.7 cells were transfected with small interference RNA for control (si-Control) or MCPIP1 (si-MCPIP1) for 24 h and then stimulated with PamCSK4 for 6 h. Three independent samples from control or MCPIP1 knocking down groups were loaded as indicated. The MCPIP1 protein level in the cell lysates was detected by Western blot with a MCPIP1 antibody (Genetex). Actin was probed as a loading control. D, HeLa cells were co-transfected with the plasmids encoding Flag-MCPIP1 and EGFP-MCPIP4. The cells were labeled with anti-Flag and visualized by Alexa Fluor594-labeled second antibody, and images were taken by confocal microscopy. E, Flag-MCPIP1 or Flag-MCPIP4 were transiently co-transfected with GFP-GW182 or GFP-Ago2 into HeLa cells. After 24 h, cells were fixed and stained with anti-Flag and visualized by confocal fluorescence microscopy. F, different combinations of expression plasmids as indicated were co-transfected with pG5luc reporter into HEK293 cells. 24 h later, cell lysates were prepared for analysis of luciferase activity. Data are presented as mean ± S.D., n = 4.