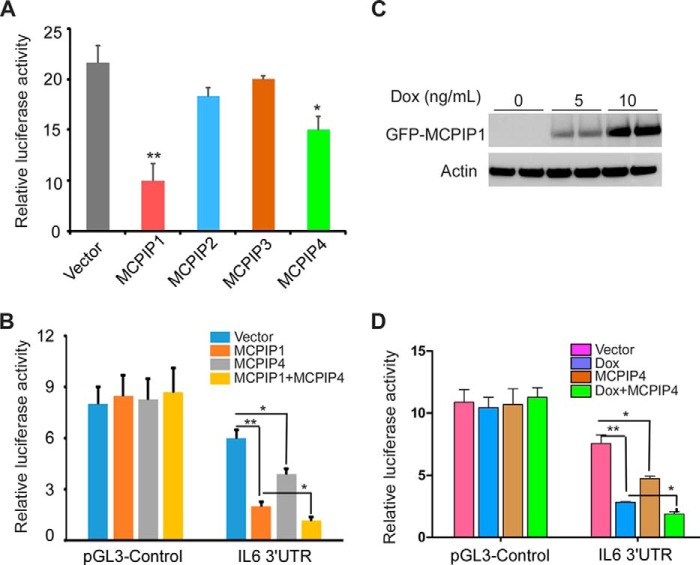
FIGURE 5.
Co-expression of MCPIP1 and MCPIP4 enhanced the repression on the reporter of IL-6 3′-UTR. A, expression plasmids of MCPIP1/2/3/4 or an empty vector were co-transfected with the luciferase reporter of IL-6 3′-UTR into HEK293 cells. A control reporter vector pRL-TK was also transfected to normalize the values. After 24 h, cell lysates were prepared, and the luciferase activity was measured by dual luciferase assay system. Data are presented as mean ± S.D., n = 4, *, p < 0.05; **, p < 0.01 versus control group. B, expression plasmids of MCPIP1 or/and MCPIP4 were co-transfected with the reporter of IL-6 3′-UTR or the pGL3-control reporter into HEK293 cells. After 24 h, the cell lysates were prepared, and the luciferase activity was measured by dual luciferase assay system. Data are presented as mean ± S.D., n = 4, *, p < 0.05; **, p < 0.01 versus vector group. C, inducible MCPIP1-stable expressed HEK293 cell line was treated with 0, 5, or 10 ng/ml of doxycycline for 24 h. The inducible expression of MCPIP1 in the cells was determined by Western blot analysis with anti-GFP antibody. Actin was probed as a loading control. D, inducible MCPIP1-stable cells were transiently co-transfected with the expression plasmid of MCPIP4 or empty vector and the reporter of IL-6 3′-UTR or the pGL3-control reporter and followed by treatment with or without 10 ng/ml of doxycycline for 24 h. The cell lysates were prepared, and the luciferase activity was measured by dual luciferase assay system. Data are presented as mean ± S.D., n = 4, *, p < 0.05; **, p < 0.01 versus vector group.