FIGURE 7.
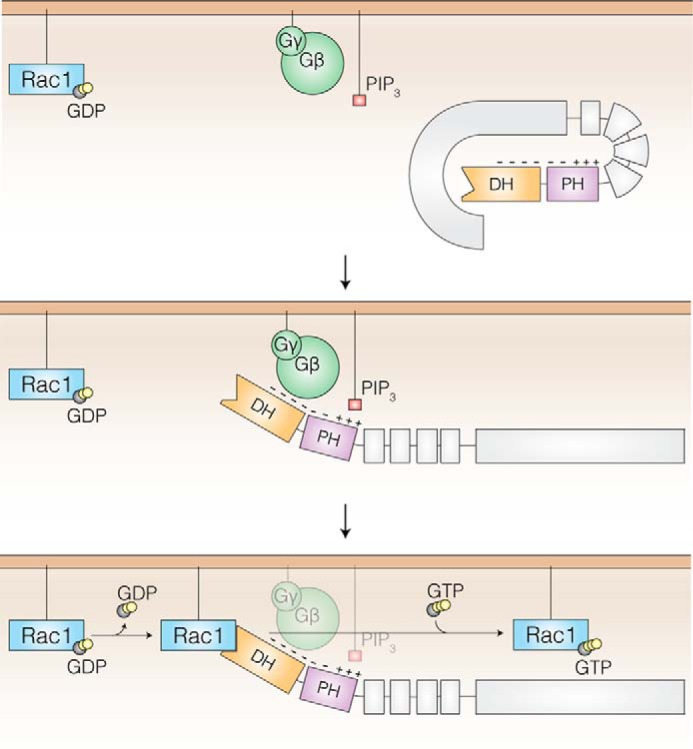
Model of Gβγ and PIP3-dependent P-Rex1 GEF activity. Low basal P-Rex1 activity is maintained through inhibitory interactions between the DH-PH tandem domain and C-terminal domains (gray) that block the GTPase binding site (DH domain, orange). At the plasma membrane, PIP3 binds via a positively charged pocket within the PH domain (purple), and Gβγ subunits bind to a negatively charged patch that spans the DH and PH domains. The binding of PIP3 and Gβγ subunits releases the C-terminal domains and exposes the Rac1 binding interface on the opposite side of P-Rex1. This allows Rac1 nucleotide exchange via the P-Rex1 DH domain.
