FIGURE 2.
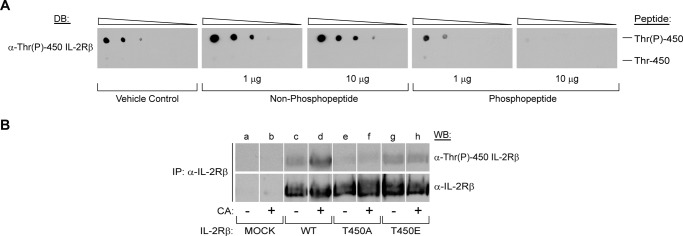
The IL-2Rβ Thr(P)-450 antibody preferentially recognizes phosphorylated peptides. A, a phosphospecific rabbit polyclonal IL-2Rβ antibody to Thr(P)-450 was generated and tested by dot blot (DB) analysis using decreasing concentrations (1 μg, 0.1 μg, 10 ng, 1 ng, and 0.1 ng) of IL-2Rβ Thr-450 (LGPPTPGVPDLVDFC) (top) and Thr-450 (LGPP(pT)PGVPDLVDFC) (bottom) peptides. For peptide competition analysis, the polyclonal Thr(P)-450 IL-2Rβ antibody was preblocked for 2 h at room temperature with vehicle control (first panel, far left), 1 or 10 μg of the non-phosphopeptide (second and third panels), or phosphopeptide (fourth and fifth panels) as indicated. B, HEK293 cells were transfected with plasmids encoding WT IL-2Rβ or the T450A or T450E mutants. 48 h post-transfection, cells were incubated in the absence (−) or presence (+) of CA (100 nm) for 60 min. IL-2Rβ was immunoprecipitated (IP), separated by SDS-PAGE, and Western-blotted as indicated. Representative data from three independent experiments are shown.
