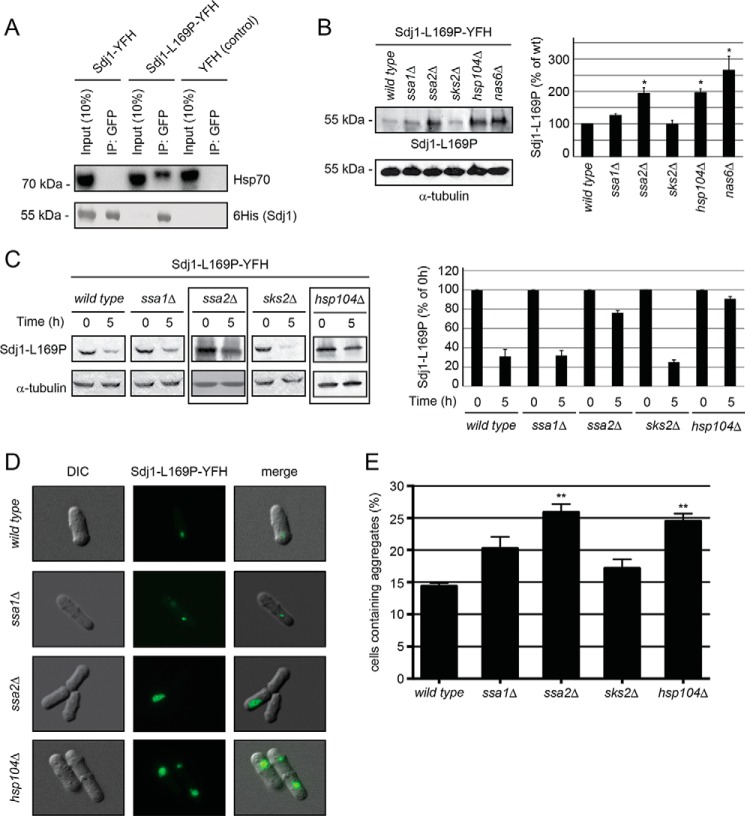
FIGURE 4.
Sdj1-L169P degradation depends on chaperones. A, immunoprecipitates (IP) from wild type S. pombe cells expressing Sdj1-YFH, Sdj1-L169P-YFH, and as a control, YFH. After adjusting the loading, the precipitated material was resolved by SDS-PAGE and analyzed by Western blotting, using antibodies to Hsp70 (upper panel) and His6 (lower panel). B, left panel, the steady-state level of Sdj1-L169P-YFH in the indicated strains was determined by Western blotting of whole cell lysates. Tubulin served as a loading control. Right panel, bar diagrams showing quantification of Western blots normalized to the wild type control. The error bars indicate the S.E. ± mean (n = 3, *, p < 0.05, Student's t test). C, left panel, the amount of Sdj1-L169P-YFH was followed in the indicated chaperone mutant strains treated with cycloheximide. Equal loading was checked using antibodies to tubulin. Note that Sdj1-L169P is stabilized in the ssa2Δ and hsp104Δ strains (boxed). Right panel, bar diagrams showing quantification of Western blots normalized to the signal at 0 h. The error bars indicate the S.E. ± mean (n = 3). D, fluorescence micrographs showing the Sdj1-L169P containing aggregates in the indicated genetic backgrounds. Note the larger aggregates in the ssa2Δ and hsp104Δ strains. E, the number of the Sdj1-L169P-YFH aggregate containing cells was determined by fluorescence microscopy for the indicated strains. The error bars indicate the S.E. ± mean (n = 3, ***, p < 0.001, Student's t test). DIC, differential interference contrast.