Abstract
Background:
Gastric cancer is the second most common cancer worldwide. In Iran, the incidence of gastric cancer is well above the world average, and is the first common cancer in Iranian men and the third one in women. Located at chromosome 14q23, SIX1 is a homolog of the Drosophila ‘sine oculis’ (so) gene and is highly conserved in numerous species. In addition to the role of SIX1 in the development, its expression is frequently dysregulated in multiple cancers. This study aimed to evaluate the clinicopathological features of the expression of SIX1 gene in gastric adenocarcinoma.
Materials and Methods:
Thirty pairs of gastric tissue samples from patients with gastric adenocarcinoma were evaluated for SIX1 gene expression using quantitative real-time polymerase chain reaction. A paired t-test or one-way ANOVA with post hoc multiple comparisons were used to analyze the differences between groups. Statistical significance was defined as P ≤ 0.05.
Results:
SIX1 expression was decreased in tumoral samples. However, its expression increased significantly in diffuse-type gastric cancer. Furthermore, there was a trend toward statistical significance in increasing SIX1 gene expression with higher grades. Of note, the difference was significant between grades I and III.
Conclusions:
The results suggest that SIX1 gene expression might be used in the future as a potential biomarker to predict the outcome of the disease as diffuse-type and grade III of gastric tumors are associated with poor prognosis.
Keywords: Diffuse-type gastric cancer, gene expression, poor prognosis SIX1, tumor grades
INTRODUCTION
Gastric cancer is the second most common cancer worldwide, with an estimated 900,000 new cases and 700,000 gastric cancer-related deaths in the world.[1] In Iran, the incidence rate of gastric cancer is well above the world average, and is the first common cancer in Iranian men and the third one in women.[2] Because of the lack of trustworthy early diagnostic methods and effective treatment, more than 80% of patients with advanced gastric cancer die of the disease or recurrent disease within 1 year after diagnosis. The majority of patients with gastric cancer are being diagnosed in advanced stages of the disease such that usual treatment protocols are ineffective in a remarkable number of cases.[3] Therefore, elucidation of the molecular characteristics of gastric tumors is an essential need to develop methods of early cancer detection and reduce its mortality.
Located at chromosome 14q23,[4] SIX1 (sineoculis homeobox homolog 1), a member of the Six gene superfamily, is a homolog of the Drosophila ‘sine oculis’ (so) gene and is highly conserved in numerous species from Drosophila to human.[5,6] The SIX1 gene product functions in concert with Eya1,[6] Pax and Dac in DNA binding[7] and regulates the expression of many downstream target genes.[8] Therefore, the SIX1 homeoprotein organizes a variety of cellular processes during normal development of some tissues and organs such as promoting progenitor cell population proliferation and their invasion before cell differentiation and specification, survival, migration and apoptosis. However, after development, expression of SIX1 changes and decreases in most normal adult tissues.[9,10,11] Furthermore, SIX1 indirectly affects cell movement and adhesion between cells and the extracellular matrix (ECM) by regulating the expression of Ezrin.[7]
In addition to the role of SIX1 in the development, its expression is frequently dysregulated in multiple cancers including breast cancer,[12] Wilms’ tumors,[13] ovarian cancer,[14] hepatocellular carcinoma,[15] alveolar rhabdomyosarcomas,[16] and cervical cancer.[17] The misexpression of SIX1 in cancer can enhance cancer cell proliferation and survival and lead to tumor inception and progression.[8,18,19] These findings explain that unsuitable SIX1 expression in adult differentiated tissues results in cell proliferation stimulus and, in turn, leads to initiation and progression of numerous cancers.[11]
Considering the dysregulation of SIX1 gene expression in various tumors, in this study, we studied the expression of SIX1 gene in gastric tumors.
MATERIALS AND METHODS
Tumor and non-tumor tissues
Thirty pairs of gastric tissue samples (tumor and their adjacent non-tumor tissues) from patients with gastric adenocarcinoma were provided from the Iran Tumoural Bank (Tehran, Iran) as described previously.[20] The clinicopathological characteristics of the specimens are shown in Table 1. Written informed consent from all subjects was obtained by the Iran Tumoral Bank. The experimental procedures were approved by the Ethics Committee of the Isfahan University of Medical Sciences. The samples were frozen in liquid nitrogen and kept at -80°C until analysis.
Table 1.
Clinicopathological parameters of gastric cancer samples

RNA isolation and reverse transcription
Total RNAs from frozen gastric cancer tissue samples were extracted using Qiazol reagent and RNeasy columns (Qiagen, Hilden, Germany) following the manufacturer's protocol. RNA integrity was examined by running on a 1% agarose gel and total RNA concentrations determined spectrophotometrically. Two micrograms of total RNA were reverse transcribed using random hexamer primers (TAG Copenhagen) and MMLV Reverse Transcriptase (Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions.
Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Synthesized cDNAs were subjected to quantitative real-time PCR using the Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Vilnius, Lithuania) and specific primers for SIX1 and TBP[21] as an endogenous control in a total volume of 20 μL reaction mixture and were run on the Rotor-gene 6000 system. The following primers were used to amplify SIX1:
SIX1 forward primer: 5’- TAAGAACCGGAGGCAAAGAG -3’
SIX1 reverse primer: 5’- AGTTTGAGCTCCTGGCGTG -3’
The amplification conditions for SIX1 were as follows: An initial denaturation step at 95° C for 10 min followed by 45 amplification cycles consisting of denaturation at 95° C for 20 s, annealing for 20 s at 55° C and an extension at 72° C for 20 s. For each sample, measurements were performed at least in triplicate. The standard curve method was used to calculate relative gene expression. For further verification of the identity of the PCR products, agarose gel electrophoresis was performed.
Statistical analysis
Data are represented as means ± standard error of mean (SEM) from at least three separate experiments. To compare the gene expression levels between the tumor and non-tumor tissues and associated clinicopathological characteristics with gene expression, Student's t test and ANOVA statistical tests were performed. The SPSS program, version 20.0, was utilized for statistical analyses, and differences were considered significant if P < 0.05.
RESULTS
SIX1 is underexpressed in human gastric adenocarcinoma tissues
Relative quantitation of the expression levels of SIX1 in gastric adenocarcinomas showed that the relative levels of SIX1 transcripts were significantly decreased (around 1.5-fold, P value = 0.018) in cancerous tissues compared with adjacent non-cancerous tissues: 0.48 ± 0.03 versus 0.64 ± 0.06, respectively, as shown in Figure 1.
Figure 1.
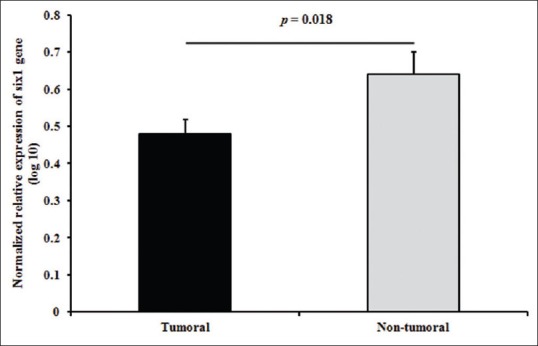
The relative expression levels of SIX1 in tumoral versus non-tumoral gastric samples. Error bars represent standard error of mean (SEM)
Association of SIX1 expression with clinicopathological parameters in gastric adenocarcinoma tissues
Next, we analyzed the association of SIX1 relative gene expression with the reported clinicopathological characteristics of the tumors (histological classifications and grade). As shown in Figure 2, the expression level of SIX1 was different in both diffuse- and intestinal-type tumors. We found that SIX1 overexpressed in diffuse-type gastric tumors (mean: 0.56) compared with intestinal-type tumors (mean: 0.40) (P value: 0.025). Furthermore, there was no significant association between the expression levels of SIX1 and different grades of the tumors (P value: 0.09). However, SIX1 was overexpressed in grade III of gastric tumors compared with grade I (P value: 0.03) [Figure 3].
Figure 2.
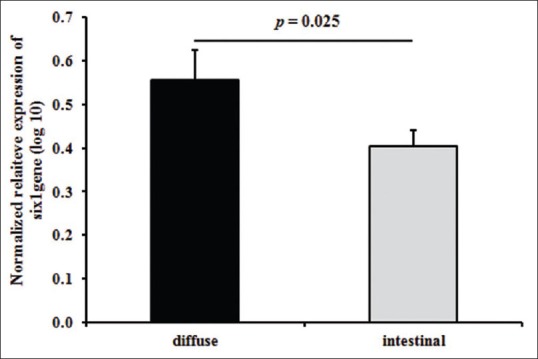
Relationship of the relative expression levels of SIX1 with the histological classifications of the gastric tumors (diffuse vs. intestinal types)
Figure 3.
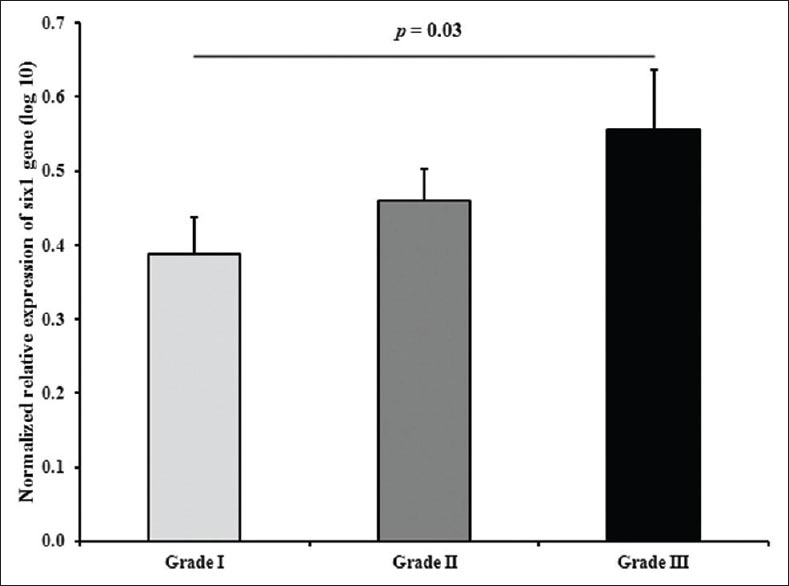
The SIX1 relative expression stratified according to different tumor grades. The difference between grades I and III was statistically significant (P = 0.03)
DISCUSSION
To the best of our knowledge, this is the first study that evaluates the expression of SIX1 gene in gastric adenocarcinoma using quantitative real-time RT-PCR. Our study showed that the relative expression of SIX1 is significantly downregulated in tumoral tissues compared with the adjacent non-tumoral tissues (P = 0.018). However, our results showed that expression of SIX1 increased significantly in diffuse-type gastric tumors in comparison with the intestinal-type gastric tumors (P = 0.025). Furthermore, SIX1 expression significantly increased in grade III gastric tumors in comparison with grade I gastric tumors (P = 0.03).
SIX1 is an important developmental regulator in several diverse tissues/organs,[10,22,23,24,25] and induces the expression of diverse genes (e.g. Cyclin D1, Cyclin A1 and c-myc) in various cell types.[8] It has been postulated that dysregulation of SIX1 leads to cancer.[7,26]
Overexpression of SIX1 has been documented in several types of cancer, including breast cancer,[12] Wilms’ tumors,[13] ovarian cancer,[14] hepatocellular carcinoma,[15] alveolar rhabdomyosarcomas[16] and cervical cancer,[17] where it facilitates proliferation and metastasis of the cancerous cells.[26] In the same vein, we observed that SIX1 expression increased significantly in diffuse type and grade III gastric cancer. Of note, microarray analyses have also shown that SIX1 overexpresses in diffuse-type gastric tumors versus intestinal-type gastric tumors.[27,28] However, Matsusaka et al. recently reported that EYA1, a Six 1 coactivator, is often methylated in both EBV + and EBV-/high methylation gastric cancers.[29] As EYA1 interacts with and functions upstream of the homeobox gene Six 1 in the development of some organs including ear and kidney,[24,30] it is plausible that SIX1 was co-underexpressed with EYA1 in gastric cancer. Furthermore, microarray analysis performed by Cui et al. showed that SIX1 expression decreases in gastric tumors versus normal gastric tissues.[31]
Located within a critical interval on chromosome 14q23, Ruf et al. identified a 3-bp deletion in the SIX1 gene in branchio-otic syndrome.[32] Furthermore, a loss of 14q23 has been reported in breast cancer,[33] gastrointestinal stromal tumors[34] and neuroblastomas.[35] In the same vein, deletions have been observed in 14q in gastric cancer.[36] Moreover, Gόmόs-Akay et al. recently reported that the most common losses in gastric adenocarcinomas were found on arms 18q (26%), 5q (21%) and 14q (21%).[37] Taken together, the overall underexpression of SIX1 in gastric cancer may be attributed to the loss of 14q.
In conclusion, this is the first report that evaluates the expression of SIX1 in gastric cancer. Our results showed that SIX1 is significantly downregulated in gastric tumors. However, our results showed that expression of SIX1 increased significantly in diffuse-type and grade III gastric tumors. Taken together, this gene might be used in the future as a potential biomarker to predict the outcome of the disease as diffuse-type and grade III gastric tumors are associated with poor prognosis.[38] Further studies should be carried out to elucidate the mechanisms which cause SIX1 underexpression in gastric tumors and to find out how SIX1 and EYA1 function in gastric cancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol. 2012;18:5164–70. doi: 10.3748/wjg.v18.i37.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 3.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 4.Boucher CA, Carey N, Edwards YH, Siciliano MJ, Johnson KJ. Cloning of the human SIX1 gene and its assignment to chromosome 14. Genomics. 1996;33:140–2. doi: 10.1006/geno.1996.0172. [DOI] [PubMed] [Google Scholar]
- 5.Seo HC, Curtiss J, Mlodzik M, Fjose A. Six class homeobox genes in drosophila belong to three distinct families and are involved in head development. Mech Dev. 1999;83:127–39. doi: 10.1016/s0925-4773(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 6.Buller C, Xu X, Marquis V, Schwanke R, Xu PX. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet. 2001;10:2775–81. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six 1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66:1982–9. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- 8.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six 1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68:2204–13. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 9.McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, et al. Six 1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009;119:2663–77. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008;101:93–126. doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 11.Kumar JP. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell Mol Life Sci. 2009;66:565–83. doi: 10.1007/s00018-008-8335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX 1: A possible mechanism of breast carcinogenesis. Proc Natl Acad Sci U S A. 1998;95:12608–13. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, et al. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–90. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, et al. Six 1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–42. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 15.Ng KT, Man K, Sun CK, Lee TK, Poon RT, Lo CM, et al. Clinicopathological significance of homeoprotein Six 1 in hepatocellular carcinoma. Br J Cancer. 2006;95:1050–5. doi: 10.1038/sj.bjc.6603399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 17.Wan F, Miao X, Quraishi I, Kennedy V, Creek KE, Pirisi L. Gene expression changes during HPV-mediated carcinogenesis: A comparison between an in vitro cell model and cervical cancer. Int J Cancer. 2008;123:32–40. doi: 10.1002/ijc.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, et al. The Six 1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101:6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang L, et al. Six 1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PLos One. 2013;8:e59203. doi: 10.1371/journal.pone.0059203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikpour P, Emadi-Baygi M, Mohammad-Hashem F, Maracy MR, Haghjooy-Javanmard S. Differential expression of ZFX gene in gastric cancer. J Biosci. 2012;37:85–90. doi: 10.1007/s12038-011-9174-2. [DOI] [PubMed] [Google Scholar]
- 21.Nikpour P, Baygi ME, Steinhoff C, Hader C, Luca AC, Mowla SJ, et al. The RNA binding protein Musashi1 regulates apoptosis, gene expression and stress granule formation in urothelial carcinoma cells. J Cell Mol Med. 2011;15:1210–24. doi: 10.1111/j.1582-4934.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Deng D, Huang H, Tian L, Chen Z, Zou Y, et al. Overexpression of Six 1 leads to retardation of myogenic differentiation in C2C12 myoblasts. Mol Biol Rep. 2013;40:217–23. doi: 10.1007/s11033-012-2052-7. [DOI] [PubMed] [Google Scholar]
- 23.Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu Px. The role of Six 1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six 1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou D, Silvius D, Fritzsch B, Xu Px. Eya1 and Six 1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–72. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abate-Shen C. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat Rev Cancer. 2002;2:777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 27.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Förster S, Gretschel S, Jons T, Yashiro M, Kemmner W. THBS4, a novel stromal molecule of diffuse-type gastric adenocarcinomas, identified by transcriptome-wide expression profiling. Mod Pathol. 2011;24:1390–403. doi: 10.1038/modpathol.2011.99. [DOI] [PubMed] [Google Scholar]
- 29.Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R, et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187–97. doi: 10.1158/0008-5472.CAN-11-1349. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six 1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox 2. Dev Cell. 2012;22:377–90. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo J, et al. An integrated transcriptomic and computational analysis for biomarker identification in gastric cancer. Nucleic Acids Res. 2011;39:1197–207. doi: 10.1093/nar/gkq960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–5. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanner MM, Karhu RA, Nupponen NN, Borg A, Baldetorp B, Pejovic T, et al. Genetic aberrations in hypodiploid breast cancer: Frequent loss of chromosome 4 and amplification of cyclin D1 oncogene. Am J Pathol. 1998;153:191–9. doi: 10.1016/S0002-9440(10)65560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Rifai W, Sarlomo-Rikala M, Andersson LC, Miettinen M, Knuutila S. High-resolution deletion mapping of chromosome 14 in stromal tumors of the gastrointestinal tract suggests two distinct tumor suppressor loci. Genes Chromosomes Cancer. 2000;27:387–91. [PubMed] [Google Scholar]
- 35.Thompson PM, Seifried BA, Kyemba SK, Jensen SJ, Guo C, Maris JM, et al. Loss of heterozygosity for chromosome 14q in neuroblastoma. Med Pediatr Oncol. 2001;36:28–31. doi: 10.1002/1096-911X(20010101)36:1<28::AID-MPO1008>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Koo SH, Kwon KC, Shin SY, Jeon YM, Park JW, Kim SH, et al. Genetic alterations of gastric cancer: Comparative genomic hybridization and fluorescence In situ hybridization studies. Cancer Genet Cytogenet. 2000;117:97–103. doi: 10.1016/s0165-4608(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 37.Gümüs -Akay G, Unal AE, Elhan AH, Bayar S, Karadayt K, Sunguroglu A, et al. DNA copy number changes in gastric adenocarcinomas: High resolution-comparative genomic hybridization study in Turkey. Arch Med Res. 2009;40:551–60. doi: 10.1016/j.arcmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: Review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


