Abstract
EEG in anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis shows generalised or predominantly frontotemporal δ–θ activity, and epileptiform potentials are less frequent than slowness. The voltage of EEG activity in this disorder is uncertain. We studied the voltage pattern of EEG of two patients. Both patients had psychiatric symptoms, central hypoventilation requiring prolonged ventilatory support, seizures, involuntary movements and autonomic instability. No patient showed abnormal findings on conventional MRI. Mature teratoma was diagnosed in one patient after ovarian tumour resection. Both patients received corticosteroids and intravenous immunoglobulins, and plasmapheresis. One patient recovered completely. In one patient, teratoma was not found, and ventilatory support or sedative drugs were given for about 35 months. The EEG voltages in both patients were decreased in all brain areas as compared with those of the healthy controls. Low-voltage EEG activity in all brain areas was evident from the psychotic stage.
Background
The severity and prognosis of acute encephalitis are usually assessed on the basis of clinical, electroencephalographic (EEG), and neuroradiological findings. Neuroradiological findings using MRI are very useful for predicting neurological outcomes. In clinical practice, however, neurologists often encounter patients with acute encephalitis who show no abnormal findings on neuroradiological studies. Low-voltage EEG activity has been proposed as a predictor of neurological outcomes in patients with viral encephalitis.1 Recently, anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis, a new form of encephalitis, has been described.2 The EEG in anti-NMDAR encephalitis shows generalised or predominantly frontotemporal δ–θ activity, and epileptiform potentials are less frequent than slowness.2 The voltage of EEG activity in this disorder is uncertain. We studied the voltage pattern of EEG of these patients.
Case presentation
The clinical features are summarised in the table 1.
Table 1.
Clinical characteristics of two patients with anti-NMDAR encephalitis.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age/sex at admission | 17/F | 46/F |
| Glasgow Coma Scale (total score) at admission | 13 | 13 |
| Clinical features | Psychiatric syndrome, seizures, involuntary movements, central hypoventilation, autonomic instability | Psychiatric syndrome, seizures, involuntary movements, central hypoventilation, autonomic instability |
| WBC in CSF (/mm3) | 81 | 60 |
| MRI abnormality | – | – |
| Postencephalitic epilepsy | – | – |
| Treatments and outcomes | ||
| Antiviral treatments | – | – |
| Immuno-treatments | ST, IVIG, PE | ST, IVIG, PE |
| Surgery | Tumour resection (teratoma) | –* |
| Duration follow-up (months) | 57 | 59 |
| Outcome | Complete recovery | Need for hospitalisation |
| EEG examination | ||
| interval from EEG to neurological onset (days) | 3 | 29 |
| Glasgow Coma Scale (total score) | 13 | 3 |
| Disease phase (3) | Psychotic | Hyperkinetic |
| Clinical features at EEG examination | Psychiatric syndrome | Seizure, involuntary movements, central hypoventilation, autonomic instability |
| Sedative drugs† | – | Propofol |
| Anticonvulsant drugs† | – | PHT, CBZ |
| Basic background wave | α | δ |
| Spike and/or sharp waves | – | – |
| Slow wave abnormalities | + | + |
| Periodic complexities | – | – |
*No tumour.
†During EEG examination.
CBZ, carbamazepine; CSF, cerebrospinal fluid; IVIG, intravenous immunoglobulin; PE, plasma exchange; PHT, phenytoin; ST, steroids; WBC, white blood cells; NMDAR, N-methyl-d-aspartate receptor.
We have recently described the detailed clinical features of one patient (patients 1; see ref. 3 for details). Both patients had psychiatric symptoms, central hypoventilation requiring prolonged ventilatory support, seizures, involuntary movements and autonomic instability. No patient showed abnormal findings on conventional MRI. Mature teratoma was diagnosed in one patient (patient 1) after ovarian tumour resection. Both patients received corticosteroids and intravenous immunoglobulins, and plasmapheresis. In summary of the timing of these treatments, patient 13 presented with unusual behaviour and delusional thinking and was admitted in a confusional state. After clinical examinations including EEG, she received intravenous acyclovir, corticosteroids and immunoglobulin. Nine days after admission, frequent generalised seizures developed, requiring ventilatory support, anticonvulsant drugs and intravenous propofol. Subsequently, the patient received plasmapheresis in addition to corticosteroids, anticonvulsant drugs and intravenous propofol. Five months after admission, she underwent a unilateral cystectomy. Patient 2 presented with unusual behaviour, delusional thinking and hallucinations, leading to admission to our hospital. She received intravenous acyclovir, corticosteroids and immunoglobulin. Twelve days after admission, frequent seizures and central hypoventilation developed. The patient received mechanical ventilation, anticonvulsant drugs and intravenous propofol. After EEG examination on day 29, she received corticosteroids, repeated doses of immunoglobulin and several courses of plasmapheresis in addition to continued treatment with sedatives and anticonvulsants. Immunocytochemical studies were performed in both patients with anti-NMDAR encephalitis, as described previously.2 In brief, HEK 293 cells were transfected with NR1 and NR2 plasmids to express NR1/NR2 heteromers. Cells expressing these heteromers showed strong reactivity with cerebrospinal fluid (CSF) in both patients, whereas non-transfected cells did not.
Investigations
EEG Recording
We studied EEGs in two clinical stages of anti-NMDAR encephalitis as previously reported.4 EEGs were obtained in psychotic (patient 1) and hyperkinetic stages (patient 2) which were available for quantitative evaluation. Since EEGs in hyperkinetic stage of patient 1 and psychotic stage of patient 2 were not available for quantitative evaluation, we studied EEGs of clinical stage of separate patient. During the hyperkinetic stage, patient 2 presented with seizures, involuntary movements and autonomic instability, which were treated with sedative and anticonvulsant drugs. We employed the international 10–20 electrode placement system for EEG (Neurofax EEG-100, Nihon Kohden, Tokyo, Japan). The presence of epileptiform potentials, slow wave abnormalities, periodic complexities and dominance of background EEG activity was visually assessed by an experienced electroencephalographist. EEG examination in patient 1 was performed 3 days after the onset of neurological symptoms (psychiatric syndrome) without any treatment, as shown in table 1. Patient 2 underwent EEG examination 29 days after the onset of neurological symptoms (psychiatric syndrome), while receiving mechanical ventilation, intravenous propofol (2.5 mg/kg/h), phenytoin (250 mg/day), carbamazepine (400 mg/day) and steroids (40 mg/day). During EEG, both patients were free of seizures and involuntary movements.
Quantitative EEG evaluation
A 90 s artefact-free EEG epoch with a stable background was selected for both patients by the same analyst. The EEG then underwent a Fast Fourier transformation with the use of a frequency analyser (QP-220A, Nihon Kohden, Tokyo, Japan), as described previously.5 The frequency ranges were divided into five bands as follows: δ (2–4 Hz), θ (4–8 Hz), α-1 (8–10 Hz), α-2 (10–13 Hz) and β (13–20 Hz). The weighted average of the wave voltage of each band was calculated within 5 s at each electrode location by the same analyser (QP-220A). The calculation was performed 18 times for the selected 90 s of EEG. The electrodes were divided into six locations as follows: frontal pole (Fp1 and Fp2), frontal (F3, F4, F7 and F8), central (C3 and C4), parietal (P3 and P4), occipital (O1 and O2) and temporal (T3, T4, T5 and T6). Quantitative EEG studies were similarly performed in sex-matched and age-matched control subjects without central nervous disorders. For statistical analysis, differences among groups were examined with the use of t test (SPSS software). p Values of less than 0.05 were considered to indicate statistical significance.
Results
Slow wave abnormalities were present in both patients, but epileptiform potentials and periodic complexes were absent during the available EEG recording in addition to other repeated EEG recording. Background EEG slowing was present in one patient. EEG showed no significant differences between the right and left sides. The EEG voltages of α-1, α-2 and β bands in both patients were decreased in all brain areas as compared with those of the healthy controls (table 2).
Table 2.
EEG voltage of each wave band in two patients with anti-N-methyl-d-aspartate receptor encephalitis
| Frontal pole median, mean | Frontal | Central | Parietal | Occipital | Temporal | |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| δ | 3.223, 3.495 | 2.246, 2.429 | 1.902, 1.868 | 1.797, 1.821 | 1.776, 1.789 | 1.572, 1.613 |
| θ | 2.165, 2.069 | 1.537, 1.625 | 1.247, 1.314 | 1.371, 1.393 | 1.525, 1.575 | 1.183, 1.247 |
| α (8–10 Hz) | 1.268, 1.289 | 1.141, 1.135 | 1.0, 1.017 | 1.095, 1.224 | 1.947, 2.026 | 1.046, 1.216 |
| α (10–13 Hz) | 1.320, 1.335 | 1.067, 1.095 | 1.019, 1.003 | 1.758, 1.695 | 3.693, 3.765 | 1.197, 1.418 |
| β | 1.062, 1.097 | 0.896, 0.914 | 0.845, 0.844 | 0.933, 0.942 | 1.246, 1.299 | 0.872, 0.880 |
| Patient 2 | ||||||
| δ | 9.837, 11.91 | 5.664, 5.922 | 5.429, 5.929 | 5.35, 5.558 | 4.962, 4.988 | 3.796, 4.109 |
| θ | 6.355, 7.242 | 3.542, 3.602 | 2.869, 3.214 | 2.631, 2.750 | 2.335, 2.455 | 2.172, 2.288 |
| α (8–10 Hz) | 1.999, 2.206 | 1.266, 1.348 | 1.196, 1.515 | 1.217, 1.286 | 1.025, 1.088 | 0.921, 1.059 |
| α (10–13 Hz) | 1.824, 1.824 | 1.050, 1.231 | 1.008, 1.410 | 1.067, 1.185 | 0.937, 1.043 | 0.974, 1.121 |
| β | 1.573, 1.790 | 1.286, 1.543 | 1.402, 2.011 | 1.514, 1.660 | 1.337, 1.431 | 1.481, 1.595 |
Low-voltage EEG activity was more evident in the psychotic stage, as compared with that in a healthy control subject (italics). Italics: significantly lower than value of healthy control (p<0.05)
Similar findings were not seen in θ or δ bands. These findings were evident in the psychotic stage rather than in the hyperkinetic stage (figure 1).
Figure 1.
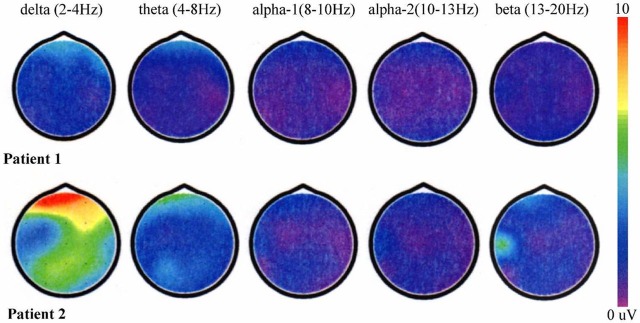
Images of the quantitative EEG voltages of δ (2–4 Hz), θ (4–8 Hz), α-1 (8–10 Hz), α-2 (10–13 Hz) and β (13–20 Hz) activities obtained by fast Fourier transformation with the use of a frequency analyser.
Outcome and follow-up
One patient (patient 1) recovered completely. In one patient (patient 2), teratoma was not found, and ventilatory support or sedative drugs were given for about 35 months.
Discussion
The most frequent finding of EEGs in acute encephalitis is the presence of diffuse slowing of background activity,6 and most EEG studies in acute encephalitis have analysed the frequencies of each wave. As compared with the healthy controls, low-voltage EEG activity was strong in the psychotic stage and the low-voltage EEG activity was weak in the hyperkinetic stage despite of the use of sedative and anticonvulsant drugs. Low-voltage EEG activity during the acute phase predicts unfavourable neurological outcomes and this is associated with lower levels of consciousness in acute encephalitis encephalitis.1 The degree of slow waves depends on the severity or the amount of cerebral involvement.7 In patients with anti-NMDAR encephalitis, the low-voltage EEG activity in all brain areas was evident from the psychotic stage.
Learning points
EEG voltages of α-1, α-2 and β bands in both patients were decreased in all brain areas.
Low-voltage EEG activity were evident in the psychotic stage rather than in the hyperkinetic stage.
In patients with anti-N-methyl-d-aspartate receptor encephalitis, the low-voltage EEG activity in all brain areas was evident from the psychotic stage.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Hosoya M, Ushiku H, Arakawa H, et al. Low-voltage activity in EEG during acute phase of encephalitis predicts unfavorable neurological outcome. Brain Dev 2002;24:161–5. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonomura Y, Kataoka H, Hara Y, et al. Clinical analysis of paraneoplastic encephalitis associated with ovarian teratoma. J Neurooncol 2007;84:287–92. [DOI] [PubMed] [Google Scholar]
- 4.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology 2008;70:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamei S, Morita A, Serizawa K, et al. Quantitative EEG analysis of executive dysfunction in Parkinson disease. J Clin Neurophysiol 2010;27:193–7. [DOI] [PubMed] [Google Scholar]
- 6.llis LS, Taylor FM. The electroencephalogram in herpes-simplex encephalitis. Lancet 1972;1:718–21. [DOI] [PubMed] [Google Scholar]
- 7.Cobb WA. Electroencephalographic changes in viral encephalitis. In: Illis LS, ed. Viral diseases of the nervous ststem. Baltimore: Williams and Wilkins, 1975:76–89. [Google Scholar]


