Figure 3.
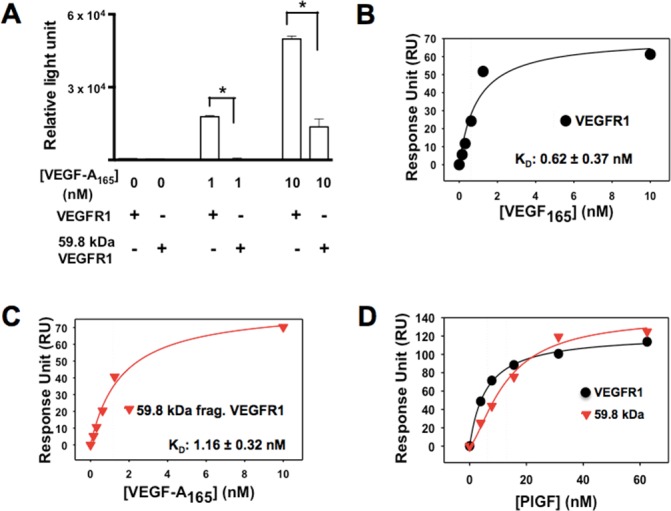
Reduced binding of VEGF-A165 to the N-terminal fragment produced by MMP14 cleavage of rmVEGFR1. (A) rmVEGFR1 and the N-terminal protein fragment produced by MMP14 cleavage of mVEGFR1 were coated onto 96-well plates and incubated with VEGF-A165 (0, 1, or 10 nM) followed by the addition of an anti-VEGF-A165-HRP. Chemiluminescence levels were analyzed with an ELISA reader to determine the binding affinity of each receptor to VEGF-A165. SPR fitting curves of VEGF-A165 with rmVEGFR1 (B) and 59.8-kDa N-terminal fragment (C) VEGF-A165 binding affinities (KD) to the immobilized rmVEGFR1 and the 59.8-kDa N-terminal VEGFR1 fragment were determined by SPR analysis using a CM5 chip. (D) SPR fitting curve of PlGF with VEGFR1 and 59.8-kDa N-terminal VEGFR1 fragment.
