Table 1. Initial Development of Aminoboration.
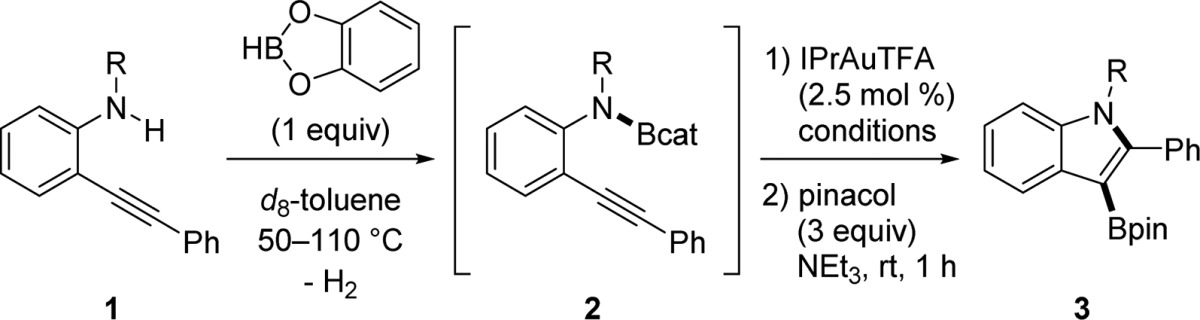
| entry | substrate | R | conditions | yield (%)a of 3 |
|---|---|---|---|---|
| 1 | 1a | H | 50 °C, 15.5 h | 0b |
| 2 | 1b | CH2Ph | 80 °C, 5 h | n.r.c |
| 3 | 1b | CH2Ph | 110 °C, 17 h | 55 |
| 4 | 1c | Ts | 50 °C, 4 h | n.r.c |
| 5 | 1c | Ts | 80 °C, 20 h | 64d |
| 6 | 1d | Mbs | 80 °C, 20 h | 66 |
Isolated yield of the Bpin product.
Only 2-phenyl-1H-indole was obtained in 69% yield.
No reaction observed as monitored by 1H NMR spectroscopy.
Average of two runs.
