Fig. 3.
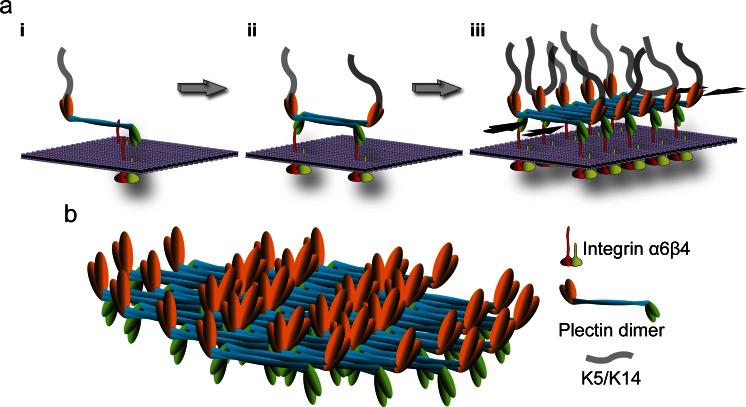
Hypothetical model of HD stabilization through plectin self-association. a Three consecutive stages (i–iii) of inner plaque formation are depicted; for simplicity, only integrin α6β4 and plectin 1a molecules are shown. In a first step (i) a parallel plectin 1a dimer binds, via its N-terminal β4-binding domains (green), to the cytoplasmic tail (red) of plasma membrane (violet)-embedded integrin β4; plectin’s C-terminal domain (orange) makes the connection to keratin filaments. In a second step (ii), two plectin 1a dimers form a tetramer by lateral association of their central rod domains in an anti-parallel fashion, creating integrin β4 and K5/K14 binding sites at both ends of the tetramer. Further self-association of plectin rod segments leads to oligomeric sheet-like structures (iii). Note that self-association of plectin 1a molecules could lead to the clustering of integrin α6β4 (as depicted), or vice versa, clustered integrins may facilitate the focal recruitment of plectin molecules promoting their alignment and lateral association; also, targeting of plectin 1a molecules to integrin β4-could occur in their keratin IF-bound (as indicated) or unbound state. Opposing black arrows, plaque-stabilizing force component provided by laterally associated rod segments. b Structural model of an inner plaque assembled by the staggered lateral association of plectin dimers. Note the focal density of potential K5/K14 and integrin β4 binding sites on opposite sides of the plate structure. This model is based on the ultrastructural analysis of paracrystalline structures formed from recombinant full-length versions of plectin’s rod domain and the lateral association potential of intact plectin molecules isolated from cells (Walko et al. 2011)