Figure 4.
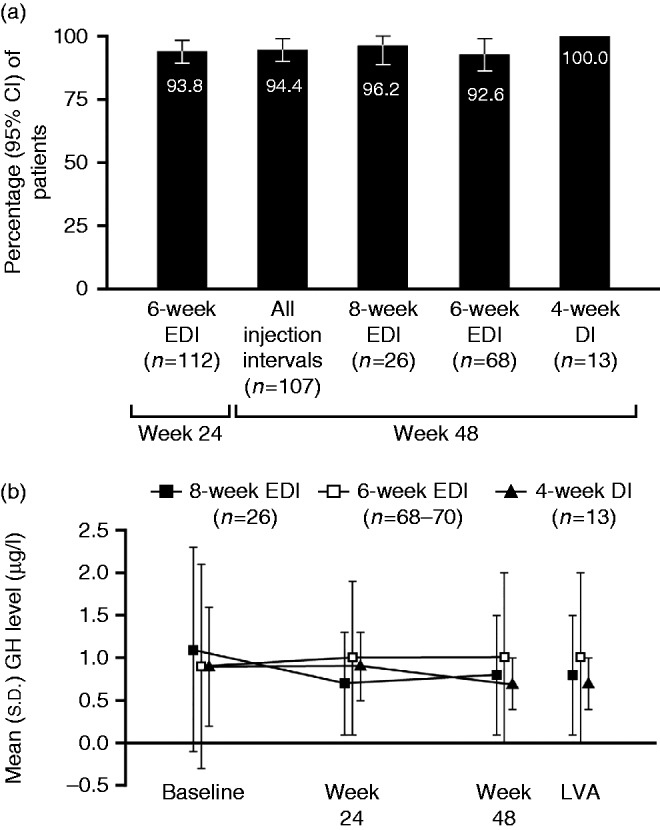
(a) Patients with GH control, showing patients with GH levels ≤2.5 μg/l at weeks 24 (end of phase 1) and 48 (end of study). (b) Serum GH levels according to allocated injection schedule for lanreotide Autogel 120 mg in phase 2. Data are from the intention-to-treat population. DI, dosing interval; EDI, extended dosing interval; GH, growth hormone.
