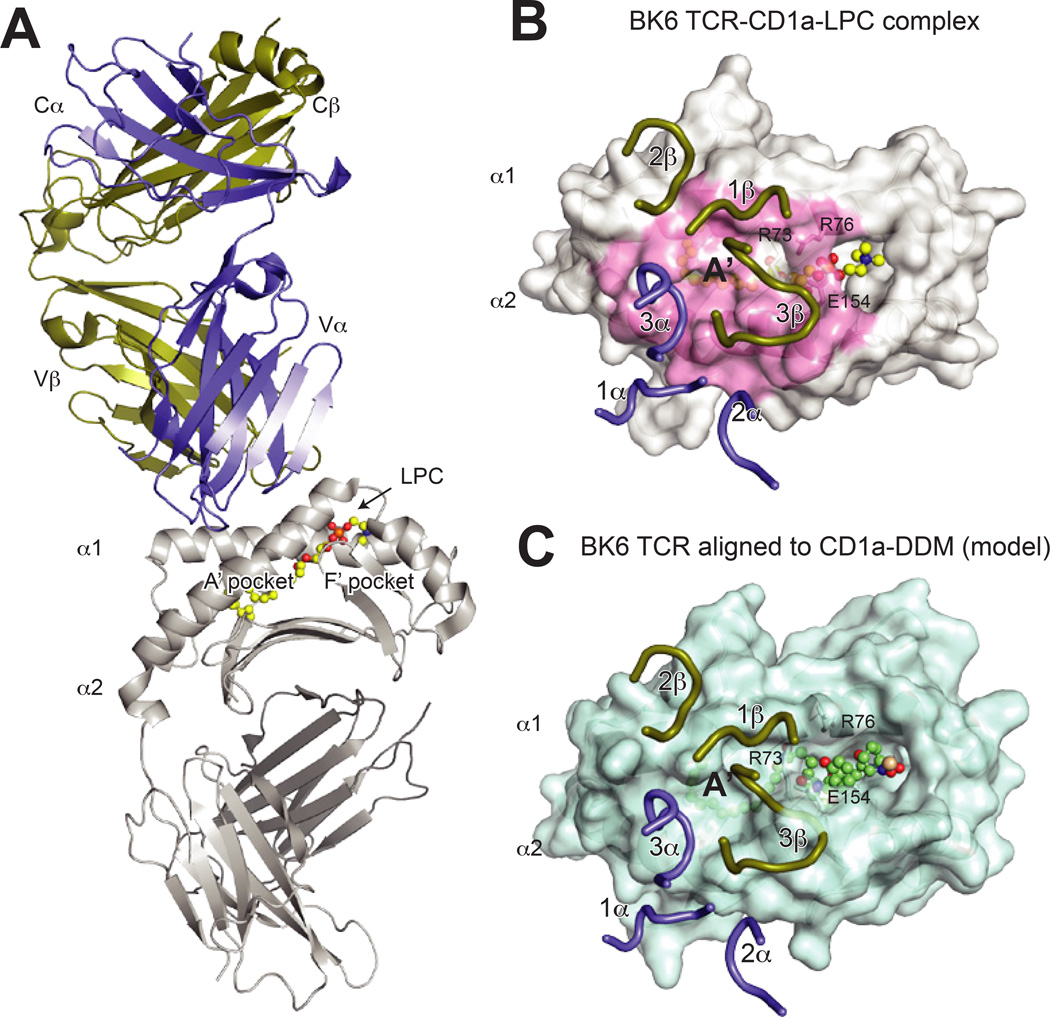
Fig. 6. Structure of the CD1a–LPC-BKT αβ and implications for lipopeptide recognition.
(A) Overall structure of the BK TCR-CD1a–lysophophatidylcholine (LPC) complex (PDB 4×6c). The TCR is shown in purple (α chain) and gold (β chain), CD1a is shown in grey and LPC is shown in yellow ball and sticks. (B) Surface view of the BK6 TCR CDR loops upon the CD1a–LPC surface. The TCR chains are colored as above, and the CD1a surface contacted by the TCR is colored in pink. Notably, the lipid head group is not contacted by the CDR loops, which instead are positioned over the A′ roof of CD1a. The residues R73, R76 and E154, which form this roof via a salt-bridge network, are indicated. (C) Alignment of the BK6-CD1a complex (aligned via CD1a) to the CD1a- didehydroxymycobactin (DDM) complex (PDB ID: 1xz0). Shown is the CD1a–DDM surface with BK6 TCR CDR loops positioned as for the CD1a–LPC structure. The larger head-group of DDM disrupts the CD1a A′ roof formed by R73, R76 and E154, and also repositions additional α helical residues, which would clash with TCR CDR loops. As a result, the contact interface as in the CD1a–LPC complex would be altered, implying that CD1a–specific TCRs specific for larger lipid species would need to undergo CDR loop conformational changes or adopt different binding modes.