Abstract
A young man presented with severe hypertension with evidence of both neurological and cardiovascular end-organ damage. Investigation revealed a small right kidney and a left renal artery aneurysm. Significant hypertension persisted even after right nephrectomy. Despite extensive investigation, no evidence was found to implicate the aneurysm in the causation of his high blood pressure. No alternative cause for hypertension was found, yet blood pressure was high even during hospital admission and observed medication dosing with eight antihypertensive agents. Sustained hypertension resulted in worsening left ventricular hypertrophy and he died suddenly at a tragically young age several years after presentation. This gentleman had truly resistant hypertension, a clinical problem which can be very difficult to manage.
Background
Uncontrolled hypertension, seemingly unresponsive to multiple medications, is a well-recognised problem in both primary and secondary care. It is commonly ascribed to patient non-compliance. True resistant hypertension, however, excludes non-adherence and white-coat hypertension. Our case illustrates the great difficulty there may be in managing such cases. Despite thorough investigation of the possible underlying mechanisms of our patient's hypertension no cause was proven and no therapy was effective. We believe this case to be a useful reminder that the management of hypertension is far from straightforward.
Case presentation
A 36-year-old white man with no history of medical problems presented to his local hospital complaining of a transient weakness and altered sensation in his right arm. He took no prescription medications, herbal remedies or recreational drugs. His mother had developed high blood pressure in her fifth decade. He was a non-smoker and there was no history of alcohol excess. The patient had undergone karyotyping during the investigation of recurrent miscarriage in a female family member. He reported that he had been identified as a carrier of a chromosomal translocation (46 XY (t(1;2) (p32;q14∼q21)). This translocation was shared by a maternal aunt and the patient's daughter, both of whom had normal blood pressure.
On examination there was right-sided facial nerve palsy. His blood pressure was 280/200 mm Hg. There were normal peripheral pulses and no arterial bruits, and there was no interarm blood pressure disparity. He was euvolaemic and there were no features of connective tissue disorders. He was not overweight; there were no other abnormal findings in the cardiorespiratory or abdominal examination. However, there was evidence of hypertensive end-organ damage, including hypertensive retinopathy (grade III) and marked left ventricular hypertrophy on echocardiography. These findings were suggestive of prior sustained hypertension.
Investigations
Biochemical measures at presentation and before any medications were startedwere: sodium 141 mmol/l, potassium 3.9 mmol/l and bicarbonate 25 mmol/l. Creatinine was 100 μmol/l; full blood count was normal and there was no proteinuria on dipstick testing.
Renal ultrasound scan showed a 13 cm left kidney that appeared normal but the right was small at 7.3 cm; a subsequent CT scan confirmed this asymmetry and a dynamic isotope renogram demonstrated that the right kidney contributed only 17% to total renal function. Formal renal angiography revealed a 10 mm saccular aneurysm of the upper pole branch of the left renal artery, with no evidence of renal artery stenosis on either side. There was no pressure gradient across the aneurysm.
Despite the normal plasma potassium and bicarbonate concentrations, measurements of random renin and aldosterone levels (unfortunately not performed until after antihypertensive medication had been started) were undertaken and recorded at 0.3 pmol/ml/h and 125 pmol/l, respectively. The aldosterone-to-renin ratio was 417 (not indicative of hyperaldosteronism). An adrenal MRI, comparing in and out-of-phase images, did not detect an adenoma. A brainstem MRI was normal, with no evidence of an arteriovenous malformation or tumour. Chest x-ray was normal and contrast CT angiogram excluded aortic coarctation.
Three urine collections on two separate occasions revealed normal levels of dopamine, epinephrine and norepinephrine metabolites. A three-day dexamethasone suppression test showed normal baseline corticosterone metabolites, with no changes in the relative proportions of metabolites to suggest Cushing's syndrome, apparent mineralocorticoid excess, glucocorticoid remediable hypertension or increased adrenal androgen production. Dexamethasone administration resulted in appropriate suppression of steroid metabolites; his blood pressure was increased slightly during the period he was taking the steroids.
Genetic analysis (performed by Professor Jeunemaitre and Dr Vargas-Poussou, HEGP, Paris) showed no mutations in the epithelial sodium channel gene, ENaC.
Differential diagnosis
Having eliminated many causes of resistant hypertension, the differential diagnosis was felt to include underperfusion of the parenchyma of the small right kidney, with renal hypoxia. This could potentially be resolved by nephrectomy, but the degree of hypertension at presentation, which failed to come under control with medication, meant that surgery carried a significant risk. Hypertension related to functional renal artery stenosis secondary to the left renal artery aneurysm also remained a possibility. Further investigation of this possibility was required, looking for evidence of ischaemic renal scarring or biochemical evidence of activation of the renin-angiotensin system, especially since operative repair of the aneurysm carried a risk of loss of renal function. Repeating the measurement of renin and aldosterone off antihypertensive medication might have helped to define the mechanism of hypertension more clearly but might have led to a further rise in blood pressure with ill-effects for the patient. Although his blood pressure remained elevated after the initiation of multiple antihypertensive medications, medication non-compliance was not thought likely by any of the clinicians involved in his care. Nevertheless, this required exclusion by supervised drug administration.
Treatment
Antihypertensive therapy was started at the time of presentation, but he remained severely hypertensive. Despite eight antihypertensive agents (methyldopa 1 g thrice daily, nifedipine LA 90 mg once daily, atenolol 100 mg once daily, losartan 100 mg once daily, perindopril 8 mg once daily, spironolactone 100 mg once daily, hydralazine 50 mg twice daily and furosemide 40 mg once daily), his clinic blood pressure readings were recorded at 205/115–230/140 mm Hg. He was admitted to hospital and resistant hypertension was confirmed during supervision of medication adherence.
A laparoscopic right native nephrectomy was performed to eliminate the possibility that the small right kidney was responsible for his hypertension; however, postoperatively he remained hypertensive (BP 200/100–210/130 mm Hg).
The question of whether there was any value in stenting/coiling of the left upper pole renal artery aneurysm in his now single kidney remained a matter of great concern, since intervention to a single renal artery might have resulted in total loss of renal function. His creatinine postnephrectomy was mildly elevated at 135 µmol/l, with normal potassium and bicarbonate levels (K 4.6 mmol/l and bicarbonate 23 mmol/l). A repeat MR renal angiogram confirmed no significant enlargement of the previously noted saccular aneurysm of the left upper pole artery had occurred since presentation. A static DMSA renogram scan showed a single normal kidney with no regional defects in perfusion to suggest scarring. Renal ultrasound showed normal resistive indices in the upper, mid-pole and lower pole arteries. Formal intra-arterial digital subtraction angiogram (IADSA) delineated the aneurysm and also demonstrated distal small vessel disease in the single left kidney (see figure 1), suggesting that there might be an intra-renal vascular substrate for continued hypertension.
Figure 1.
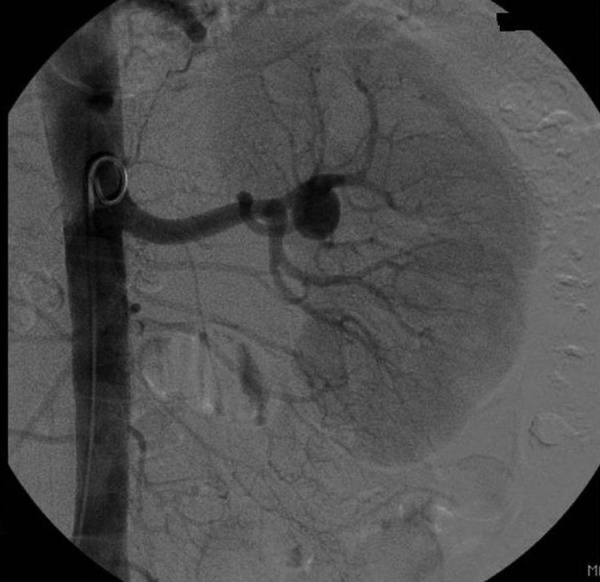
Selective left renal angiogram showing saccular aneurysm of the left upper pole renal artery.
He underwent selective upper and lower pole renal vein sampling for renin and aldosterone. Due to the severity of his hypertension this test was performed while receiving his usual antihypertensive medication (table 1).
Table 1.
Results of selective renal vein sampling: normal ranges are shown
| Renin (0.2–2.2 pmol/ml/h) | Aldosterone (135–400 pmol/l) | |
|---|---|---|
| Upper pole renal vein | 0.3 | 126 |
| Lower pole renal vein | 0.4 | 143 |
| IVC above renal vein | 0.6 | 130 |
| IVC below renal vein | 0.3 | 124 |
IVC, inferior vena cava.
There were no significant differences in regional renin or aldosterone levels to suggest that the cause of his hypertension was hyper-reninism secondary to a functionally relevant renal artery stenosis. In the absence of a perfusion defect on functional imaging or biochemical evidence of an elevated renin level or a pressure gradient across the aneurysm, intervention was not thought to be indicated.
At this stage our patient continued to have very high blood pressures and it was still not clear what was driving such treatment-resistant hypertension. Since many of his early investigations had been performed while he was taking antihypertensive medications, he was admitted for observation and intra-arterial blood pressure monitoring while his antihypertensive agents were gradually withdrawn. Off all antihypertensive medication blood pressure recordings rose to 220/140 mm Hg with headaches. Recumbent plasma renin and aldosterone were measured while he was free from all medications; levels were 1.35 pmol/ml/h and 580 pmol/l (slightly raised), respectively. Urinary steroid analysis before and after dexamethasone suppression remained normal. Twenty-four hour urinary electrolytes were as follows: sodium 68 mmol, potassium 39 mmol, magnesium 12 mmol. Thus he had mild hyperaldosteronism (with persistence of high blood pressure when on spironolactone therapy). He had a low urinary sodium excretion, but no genetic evidence of Liddle's syndrome, and he lacked the classical presentation with a hypokalaemic metabolic alkalosis seen in patients with Liddle's syndrome. A trial of amiloride did not have a significant sustained effect on blood pressure control.
Outcome and follow-up
Blood pressure remained very high (200/100 mm Hg) despite continued adherence to therapy. Within 4 years of his original presentation he developed exertional chest and left arm pain. Intra-arterial manometry at the time of coronary angiography confirmed severe central and peripheral hypertension, with no steps in pressure to suggest a coarctation. A 40–50% stenosis of the left anterior descending artery was identified, together with profound left ventricular hypertrophy.
Eight years after his original presentation he collapsed at home and sadly died. Postmortem investigation indicated the likely cause of death to be an arrhythmia secondary to his very severe left ventricular hypertrophy. No renal artery stenosis or aneurysm rupture was identified.
Discussion
This patient illustrates the reality of resistant hypertension and the need for a systematic approach to its investigation and management. While his abnormal renal anatomy may have been an underlying factor, the reason for his lack of response to multiple and revised antihypertensive agents remains unclear. Compliance with medication is a common cause of treatment failure1 but was never in question with this patient; severe hypertension was confirmed on every inpatient stay despite supervised drug administration.
The surgical literature regarding the repair of renal artery aneurysms suggests an improvement in hypertension in approximately 50% of patients,2 but it is difficult to predict which patients will experience improved blood pressure. Even when there is no anatomical renal artery stenosis, blood pressure can still improve postoperatively. The mechanism of hypertension is thought to be related to disordered flow around the aneurysm or perhaps distal microembolisation.3 In our patient the lack of biochemical or imaging evidence of functional obstruction by the aneurysm argued against attempting aneurysm repair.
The patient was known to be a carrier of a translocation 46 XY (t(1;2) (p32;q14∼q21)). Other family members carried this translocation and were normotensive, yet it is still tempting to speculate that it bears some relationship to this patient's resistant hypertension. However, none of the known Mendelian genes involved in hypertension are located in this area4 and none of the so-far-described single-nucleotide polymorphisms associated with hypertension have an effect size that could explain the severity of hypertension seen in this patient.5
We did not explore the possibility of novel antihypertensive therapies such as selective renal denervation6 or baroreceptor modulation7 in our patient. The role of neural mechanisms in the control of blood pressure may have been important in his case as there is evidence that even a small, unilateral focus of renal damage may be sufficient to increase sympathetic activation8; this was not investigated in our patient. Perhaps these strategies might have might have offered the potential for better control of blood pressure than the pharmacological treatment that was provided. Recent NICE guidelines9 suggest the use of renal denervation is a valid approach to the management of resistant hypertension, when performed in the appropriate setting. Several case reports10 11 have documented a reduction in blood pressure following insertion of periaqueductal grey matter electrodes (for the management of chronic pain) that appeared to be independent of the degree of pain relief obtained, offering another approach to blood pressure reduction.
Despite the many advances in our understanding of the dysregulation of blood pressure control it is apparent from this case that there is still more that we need to learn. Perhaps a renewed focus on neurogenic mechanisms involving the kidney and central nervous system may provide answers for some patients who currently live with uncontrolled hypertension.
Learning points.
Truly resistant hypertension demands thorough investigation of potential endocrine, anatomical and genetic causes.
Medication non-compliance should be formally excluded in difficult cases like this.
Patients with resistant blood pressure should receive specialist investigation and care, including consideration of novel therapeutic strategies, since uncontrolled severe hypertension leads to severe morbidity and mortality.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens 2006;19:1190–6. [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer T, Reiher L, Grabitz K, et al. Reconstruction for renal artery aneurysm: operative techniques and long-term results. J Vasc Surg 2003;37:293–300. [DOI] [PubMed] [Google Scholar]
- 3.Henke P, Cardneau J, Welling TR, et al. Renal artery aneurysms: a 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg 2001;234:454–62. discussion 62–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luft F. Mendelian forms of human hypertension and mechanisms of disease. Clin Med Res 2003;1:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Shaughnessy K. Dissecting complex traits: recent advances in hypertension genomics. Genome Med 2009;1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–9. [DOI] [PubMed] [Google Scholar]
- 7.Scheffers IJ, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol 2010;56:1254–8. [DOI] [PubMed] [Google Scholar]
- 8.Ye S, Gamburd M, Mozayeni P, et al. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens 1998;11(6 Pt 1):723–8. [DOI] [PubMed] [Google Scholar]
- 9.Percutaneous transluminal radiofrequency sympathetic denervation of the renal artery for resistant hypertension. http://www.nice.org.uk/ipg418 (accessed 10 Apr 2012)
- 10.Javed S, McBryde F, Khan S, et al. 182 Deep Brain Stimulation (DBS) Surgery for treatment-resistant hypertension: a promising solution to a silent killer. Neurosurgery 2012;71:E571. [Google Scholar]
- 11.Pereira EA, Wang S, Paterson DJ, et al. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci 2010;17:124–7. [DOI] [PubMed] [Google Scholar]


