Abstract
A 51-year-old Japanese woman developed systemic lupus erythematosus (SLE) in 1995. In August 2005, she had massive pericardial effusion due to lupus pericarditis, which was compromising her circulation. Methylprednisolone pulse, intravenous cyclophosphamide pulse and pericardiocentesis were all ineffective. The pericardium was cut surgically to create a passage to drain the liquid into the pleural cavity. The procedure was temporarily effective; however, massive liquid accumulated in the pleural cavity within 1 year. Oral tacrolimus and topical betamethasone injection were ineffective. Since the interleukin-6 (IL-6) level in the effusion was markedly increased (1160 pg/ml), tocilizumab was administered intravenously at a dose of 8 mg/kg every 4 weeks. The effect was astonishing and only a residual amount of pericardial effusion remained. Prednisolone was tapered successfully from 15 to 5 mg daily. Tocilizumab is a treatment of choice when we confront an intractable serositis with massive effusion in SLE, if the IL-6 level is high.
Background
There are no previous reports on tocilizumab for treatment of pericardial effusion in patients with lupus pericarditis.
Systemic lupus erythematosus (SLE) causes injuries to various organs through an autoimmune mechanism, of which pericarditis is a frequent complication. A combined series reported clinically evident pericarditis in approximately 28% of patients with SLE, whereas a combined autopsy series revealed pericarditis in 65% of patients with SLE1; cardiac tamponade is very rare.2 Pericarditis and accumulation of pericardial effusion in SLE usually respond well to glucocorticoids and seldom require immunosuppressants such as cyclophosphamide.
We previously reported massive intractable pericardial effusion in a patient with lupus pericarditis, in which methylprednisolonepulse, intravenous cyclophosphamidepulse and pericardiocentesis were ineffective. The pericardium needed to be cut surgically to drain the fluid into the left pleural space.3 It was effective for approximately 1 year, after which massive pericardial effusion accumulated in the left pleural space compromising respiration. The case reported here is the same case mentioned above, in which massive effusion in the left pleural space improved with tocilizumab.
Case presentation
The patient was a 51-year-old Japanese woman who was diagnosed with SLE with pericarditis and lupus nephritis in 1995. The accumulation of fluid was so enormous as to cause cardiac tamponade (figure 1A); pericardial excision to drain the effusion to pleural space was mandatory to improve the condition (figure 1B). Up to this clinical point, the details were published in a separate article.3
Figure 1.

Chest radiographs showing pericardial effusion decreased after placement of fenestration into the pericardium. (A) Before pericardial fenestration, (B) after pericardial fenestration.
Although pericardial fenestration had been effective for approximately 1 year, fluid gradually reaccumulated in the pleural space and drainage through pleurocentesis needed to be repeated. Tacrolimus (3 mg/day for 6 weeks), intra-pleural glucocorticoid (betamethasone 10 mg performed once), or cyclophosphamide-pulse (500 mg infused twice 2 weeks apart) was ineffective.
In December 2008, her chest radiograph showed a very high amount of pericardial effusion in her left pleural space, which necessitated emergency admission to our hospital (figure 2A). On admission, her blood pressure and pulse rate were 124/86 mm Hg and 90 bpm (regular), respectively. Heart and breath sounds were normal. Laboratory data on admission were as follows: haemoglobin, 15.0 g/dl; platelets, 31.9×104/μl; white blood cells, 10 900/μl (neutrophils 79%, lymphocytes 17%, monocytes 4%); fibrin and fibrinogen degradation product, 1.6 μg/ml; D-dimer, 0.7 μg/ml; normal transaminase levels; lactate dehydrogenase, 272 mU/ml; blood urea nitrogen, 10 mg/dl; creatinine, 0.39 mg/dl; C reactive protein, 0.06 mg/dl; C3, 65 mg/dl and C4, 25 mg/dl. Antidouble-stranded DNA antibody was weakly positive at 9.1 IU/ml. Urinalysis showed no abnormalities. Pericardial effusion was tested negative for bacteria, fungi or acid-fast bacteria in culture (table 1). The level of interleukin-6 (IL-6) in pericardial effusion was markedly elevated at 1160 pg/ml, whereas that in the serum was 6.1 pg/ml.
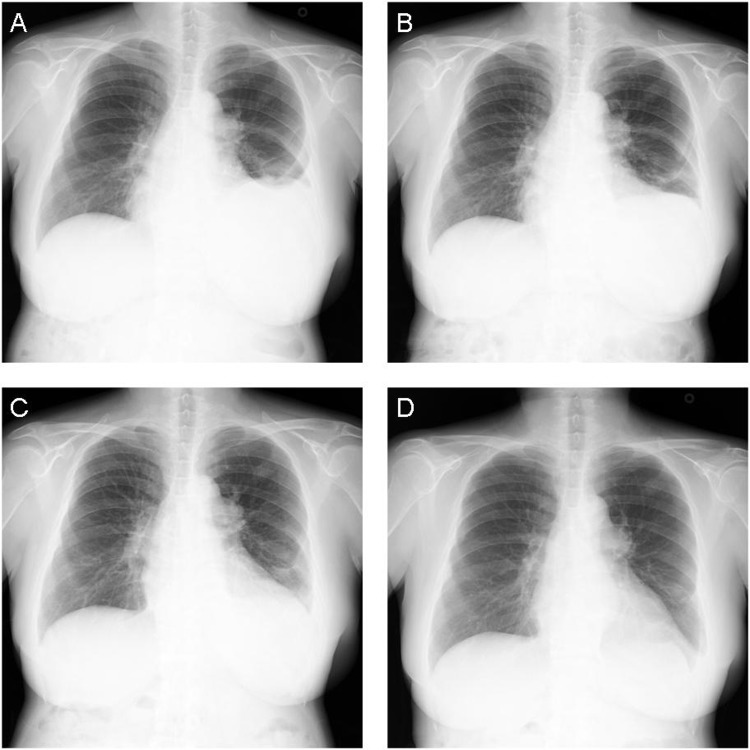
Figure 2.
Chest radiographs showing a gradual decrease in pericardial effusion after tocilizumab treatment. (A) Before tocilizumab treatment, (B) 1 month, (C) 6 months and (D) 1 year after tocilizumab treatment.
Table 1.
Characteristics of pericardial fluid
| Colour | Turbid |
|---|---|
| pH | 8.0 |
| Cell number | 50/μl |
| Neutrophil | 6% |
| Monocytoid cell | 16% |
| Lymphocyte | 66% |
| Mesothelial cell | 12% |
| Protein | 3.5 g/dl |
| Glucose | 0.11 g/dl |
| Lactate dehydrogenase | 126 mU/dl |
| Adenosine deaminase | 10.6 IU/l |
| Interleukin-6 | 1160 pg/ml |
| Bacteriology | |
| Bacteria including acid-fast | Negative |
| Fungi | Negative |
| Polymerase chain reaction for—Mycobacterium tuberculosis | Negative |
| Cytology | Class 1 |
Treatment
Owing to the tremendously high concentration of IL-6 in pericardial effusion and unresponsiveness to conventional treatment, intravenous administration of tocilizumab was tried at a dose of 8 mg/kg every 4 weeks, similar to that for rheumatoid arthritis, in January 2009.
Outcome and follow-up
Chest radiographs revealed that the amount of pericardial effusion began to decrease 1 month after treatment with tocilizumab (figure 2B), and was markedly decreased after 6 months (figure 2C). Only a small amount of pericardial effusion was found after 1 year (figures 2D and 3A), and almost no effusion was detected by chest radiographs 1.5 years after treatment (figure 3B).
Figure 3.
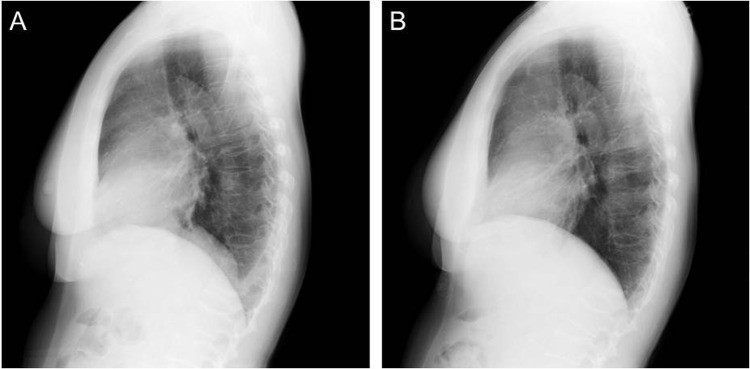
Lateral view of chest radiographs showing fluid in pleural space after tocilizumab treatment. (A) After 13 months and (B) after 1.5 years of treatment.
The infusion-interval of tocilizumab was prolonged from 4 to 5 weeks at 19 months after the start of tocilizumab, to 6 weeks after 22 months, to 7 weeks after 2 years and to 8 weeks after 26 months. Prednisolone, which was given at a dose of 15 mg/day when tocilizumab was started, was successfully tapered to 5 mg/day. No pericardial effusion was observed on chest radiographs 2.5 years after treatment.
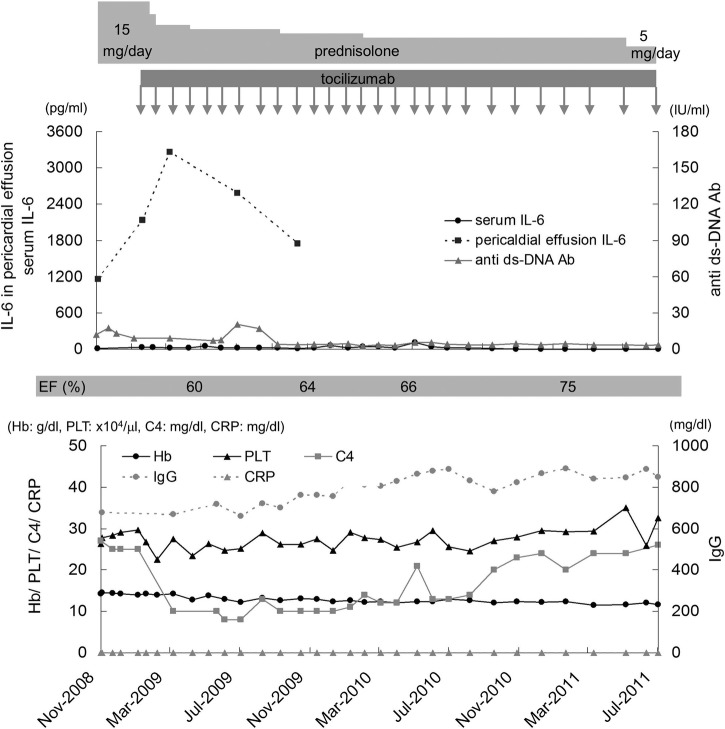
Tocilizumab showed no significant changes in the markers of SLE, such as anti-double-stranded DNA antibodies, and C3/C4. The IL-6 level in pericardial effusion temporarily increased to 3260 pg/ml at 2 months after tocilizumab treatment and subsequently decreased to 1750 pg/ml after 10 months. Thereafter, the amount of pericardial fluid was too small to collect for IL-6 measurement. Meanwhile, the serum IL-6 level, which was 6.1 pg/ml before tocilizumab administration, increased to 28.7 pg/ml 1 week after treatment and fluctuated between 13.9 and 108 pg/ml over the course of the next 1.5 years. Then, it steadily decreased and remained within the normal range (≤4.0 pg/ml; figure 4).
Figure 4.
Clinical course of the present case.
Discussion
Tocilizumab is a humanised antihuman IL-6 receptor monoclonal antibody.4 Tocilizumab binds to soluble IL-6 receptors present in serum and joint fluid as well as membrane-bound IL-6 receptors expressed on the surface of cells, leading to inhibition of receptor-mediated IL-6 signalling and suppression of various physiological activities of IL-6.5 IL-6 is a physiologically important substance involved in an immune response as well as inflammation. Abnormal continuous production of IL-6 is associated with pathogenesis of various autoimmune disorders.6 The efficacy of tocilizumab has been reported in the treatment of autoimmune diseases, including rheumatoid arthritis (RA), juvenile idiopathic arthritis and Castleman's disease.7–12 There was no finding suggestive of concomitant Castleman's disease, such as fever, rash, lymphadenopathy, interstitial pneumonia, nephritis, increased acute-phase protein or hypergammaglobulinaemia.
In the present case, tocilizumab was administered at a dose of 8 mg/kg to treat massive pericardial effusion due to chronic lupus pericarditis that was unresponsive to conventional treatment and pericardial fenestration. The amount of pericardial effusion was markedly decreased; the dose of prednisolone was successfully tapered; and the infusion-interval of tocilizumab was prolonged every 8 weeks.
In a previous report on the efficacy of tocilizumab for SLE, systemic lupus erythematosus disease activity index scores and systemic lupus activity measure scores significantly improved at week 14 of tocilizumab treatment when compared with those pretreatment.13 There are no previous reports on tocilizumab for treatment of pericardial effusion in patients with lupus pericarditis. In the present case, treatment with tocilizumab resulted in a remarkable reduction in pericardial effusion, indicating that IL-6 might have played a central role in the formation of massive pericardial effusion.
In a study by Nishimoto et al, when tocilizumab was administered in patients with rheumatoid arthritis and Castleman's disease, serum IL-6 levels markedly increased, peaked at about 4 weeks after administration and then decreased. They also found that the increase in serum IL-6 after tocilizumab infusion was greater in patients with higher disease activity, suggesting that an increased level of free IL-6 may represent true disease activity.14
In the present case, a 4.7-fold increase in serum IL-6 level was observed 1 week after tocilizumab infusion compared with the pretreatment level (from 6.1 to 28.7 pg/ml). Although the IL-6 level fluctuated during the next 1.5 years, it decreased and remained within the normal range thereafter. The decrease in serum IL-6 level paralleled that in pericardial effusion. However, as shown in figure 4, the level of serum IL-6 was quite low compared with that in pleural effusion, which might account for the low level of serum CRP even at the time when massive pleural effusion accumulated. Tocilizumab did not show significant changes in the levels of antidouble-stranded DNA antibodies, C3 and C4. This indicates that pathogenesis of serositis might be different from the abnormal level of humoral immunity; serositis in the present case is more dependent on the level of topical IL-6.
The levels of CRP in pericardial effusion were low at 0.52 mg/dl before tocilizumab administration, at 0.20 mg/dl 8 days after the start of tocilizumab administration, and at or below the measureable limit of 0.05 mg/dl 2 months after the start and thereafter. Although the levels of serum CRP and serum IL-6 were extremely low as shown in figure 4, the level of IL-6 in pericardial effusion was markedly high. This suggested that IL-6 in pericardial effusion is locally produced by the epicardium.
IL-6 level in the pericardial fluid is elevated in patients with pericarditis associated with SLE and RA.15 16 However, this is the first report where tocilizumab was used for treatment of pericarditis associated with SLE.
Learning point.
Therapy with tocilizumab is a treatment of choice when we are confronted with patients having intractable serositis and high interleukin-6 levels in effusion.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: None.
References
- 1.Moder KG, Miller TD, Tazelaar HD. Cardiac involvement in systemic lupus erythematosus. Mayo Clin Proc 1999;74:275–84. [DOI] [PubMed] [Google Scholar]
- 2.Castier MB, Albuquerque EM, Menezes ME, et al. Cardiac tamponade in systemic lupus erythematosus. Report of four cases. Arq Bras Cardiol 2000;75:446–8. [DOI] [PubMed] [Google Scholar]
- 3.Kamata Y, Iwamoto M, Aoki Y, et al. Massive intractable pericardial effusion in a patient with systemic lupus erythematosus treated successfully with pericardial fenestration alone. Lupus 2008;17:1033–5. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Tsuchiya M, Saldanha J, et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res 1993;53:851–6. [PubMed] [Google Scholar]
- 5.Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 2005;5:1731–40. [DOI] [PubMed] [Google Scholar]
- 6.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol 2006;2:619–26. [DOI] [PubMed] [Google Scholar]
- 7.Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 2009;19:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007;66:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto J, Garnero P, van der Heijde D, et al. Humanized anti-interleukin-6-receptor antibody (tocilizumab) monotherapy is more effective in slowing radiographic progression in patients with rheumatoid arthritis at high baseline risk for structural damage evaluated with levels of biomarkers, radiography, and BMI: data from the SAMURAI study. Mod Rheumatol 2011;21:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 2008;371:998–1006. [DOI] [PubMed] [Google Scholar]
- 11.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 2005;106:2627–32. [DOI] [PubMed] [Google Scholar]
- 12.Ogata A, Tanaka T. Successful treatment of reactive arthritis with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Int J Rheumatol 2012;2012:946048. [DOI] [PubMed] [Google Scholar]
- 13.Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010;62:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008;112:3959–64. [DOI] [PubMed] [Google Scholar]
- 15.Vila LM, Rivera del Rio JR. Lymphocyte populations and cytokine concentrations in pericardial fluid from a systemic lupus erythematosus patient with cardiac tamponade. Ann Rheum Dis 1999;58:720–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki Y, Tanaka A, Shimamoto K, et al. A case of rheumatoid pericarditis associated with a high IL-6 titer in the pericardial fluid and tocilizumab treatment. Mod Rheumatol 2011;21:302–4. [DOI] [PubMed] [Google Scholar]