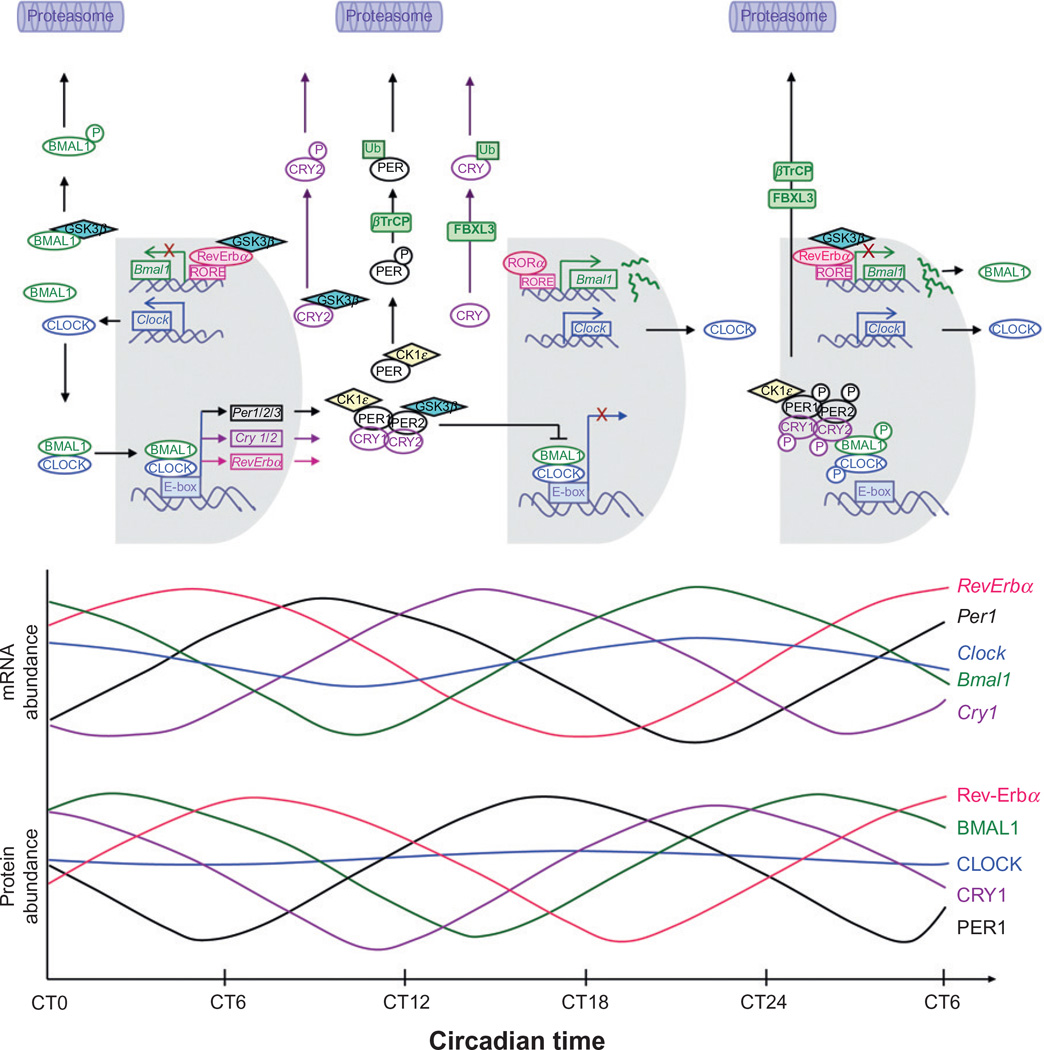
Figure 9.4.
Mammalian molecular clock and oscillation of mRNA and protein in peripheral tissues. The molecular clock is regulated by interacting transcription–translation feedback loops. As depicted in this figure, the expression of components of the molecular clock are determined by the balance of synthetic versus degradative processes. Specifically, the circadian cycle starts with the accumulation of the BMAL1 protein in the cytoplasm (CT15–CT3). As BMAL1 accumulates to sufficient levels in the cytoplasm, it forms heterodimers with CLOCK, which is constitutively present over the circadian cycle. Nondimerized BMAL1 is targeted by the kinase GSK3β, for phosphorylation and subsequent degradation. Upon dimerization, the CLOCK: BMAL1 heterodimer translocates to the nucleus where it binds to E-box sequences in the promoter region of the negative regulator genes (Per, Cry, RevErba) and induces their transcription. This leads to peaks in the mRNA levels of Per1, Per2, Per3, Cry1, Cry2, and RevErba between CT2 and CT17 followed by peaks in their protein levels 4–6 h later. In the cytoplasm, the negative regulators form multimeric complexes that translocate to the nucleus to inhibit the transcriptional activity of the CLOCK:BMAL1 heterodimer. The kinetics of this interaction depends on the availability of the negative regulators. Free CRY and PER are targeted by GSK3β and CK1ε respectively for phosphorylation and subsequent degradation via the proteasomal pathway. At around CT15–CT20, RORα binds to RORE elements in the promoter region of Bmal1 and induces Bmal1 transcription. This allows for the accumulation of Bmal1 mRNA with a peak at around CT21. Meanwhile, CK1ε phosphorylates all members of the PER/CRY complex as well as CLOCK and BMAL1. This hyperphosphorylated complex is shuttled to the proteasome for degradation between CT21 and CT24, freeing the E-box site for another round of CLOCK:BMAL1 binding. The delay imposed by transcription, translation, and posttranslational modifications is what defines the period of circadian rhythms (Lee et al., 2001; Lowrey and Takahashi, 2004; Sahar et al., 2010; Shearman et al., 2000; Ueda et al., 2002).