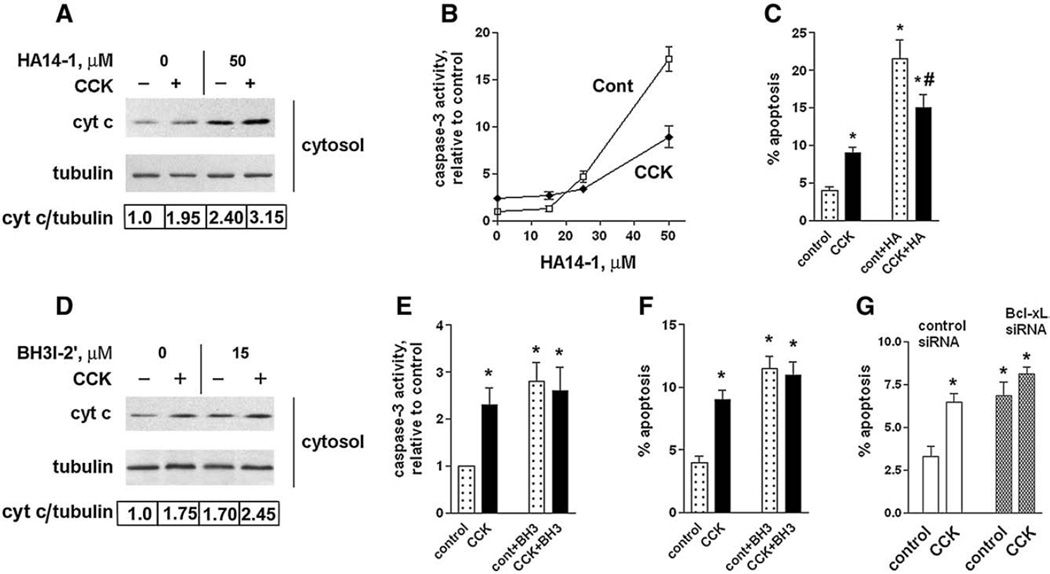
Fig. 7.
Bcl-xL/Bcl-2 inhibitors induce cytochrome c release, caspase-3 activation, and apoptosis in pancreatic acinar cells. These effects are less in CCK-hyperstimulated acinar cells than in unstimulated cells. (A–F) Rat pancreatic acinar cells were incubated for 3 h with and without 100 nM CCK-8 and indicated concentrations of HA14-1 (HA) or 15 µM BH3I-2′ (BH3). (G) Mouse pancreatic acinar cells were cultured 24 h in DMEM medium, as described in Materials and methods, transfected with control or Bcl-xL siRNA, and then incubated for 3 h in 199 medium with and without 100 nM CCK-8. (A D). Cytochrome c levels were measured in cytosolic fractions by immunobloting. Blots were re-probed for α-tubulin to confirm equal protein loading. The intensities of cytosolic cytochrome c and α-tubulin bands in the same sample were densitometrically quantified, and their ratio normalized to that in control (i.e., cells without CCK and the inhibitors) and presented below the blots. (B, E). Caspase-3 activity was measured by a fluorogenic assay using DEVD-AMC as a substrate. (C, F, G). Apoptosis was measured by the percentage of cells with apoptotic nuclear morphology using Hoechst 33258 staining. For each condition, at least 1000 cells were counted in 3 different acinar cell preparations. The inhibitor concentrations used were 50 µM HA14-1 (HA) or 15 µM BH3I-2′ (BH3). Values are means ± SE (n = 3). *p< 0.05 compared to cells incubated without CCK in the absence of the inhibitor. #p< 0.05 compared to cells incubated with CCK in the absence of the inhibitor.