Figure 4. Tet1-deficient hematopoietic stem display increased self-renewal in vivo with a bias toward B cell differentiation.
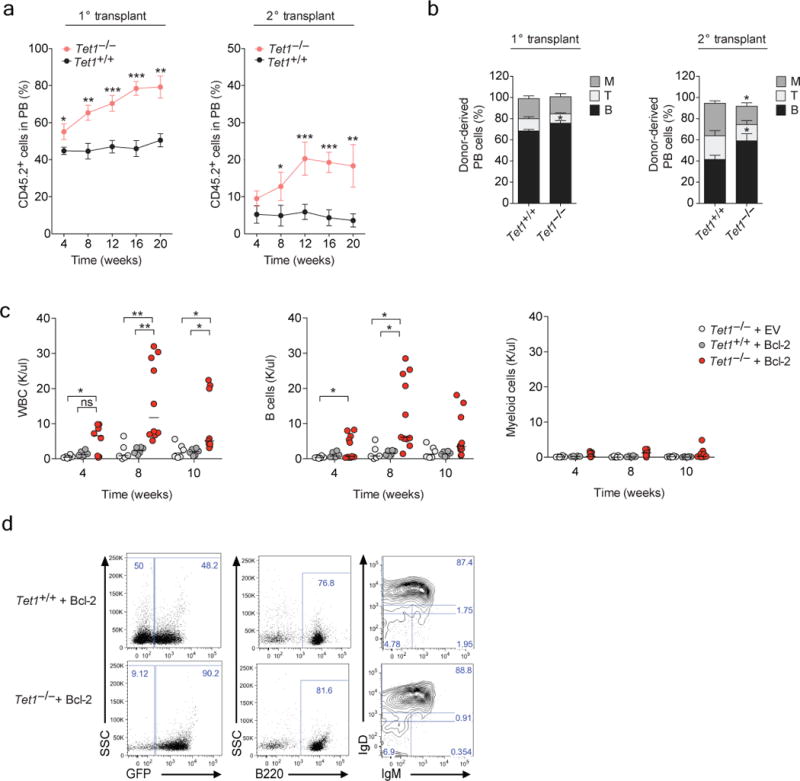
Competitive bone marrow (BM) reconstitution assays. Primary transplants were performed with CD45.2+ Tet1+/+ and Tet1−/− total BM cells (200,000 per mouse), mixed in equal ratio with CD45.1+ support BM cells and transplanted into lethally irradiated recipient mice. 20 weeks post transplant, CD45.2+ Tet1+/+ and Tet1−/− purified LT-HSCs (500 per mouse) were serially transplanted into lethally irradiated recipient mice with CD45.1+ support BM. a) Frequency of donor-derived CD45.2+ cells in the peripheral blood (PB) of primary and secondary transplanted mice. Data are the average of two independent experiments (mean ± SEM, n = 3 recipient mice per donor BM), n = 2 donor BM per genotype, per experiment. b) Average frequency of CD45.2+Lineage+ cells stained for CD11b/Gr1 (M), B220 (B) and CD3 (T) surface markers in peripheral blood 20-weeks post transplant in secondary recipient mice (mean ± SEM, n = 6 mice per genotype). Loss of Tet1 cooperates with Bcl2 overexpression to drive B lymphocytosis in mice. Purified LSK cells from Tet1+/+ and Tet1−/− mice were transduced with either pMIG-Bcl2 or an empty vector control retrovirus, and transplanted into lethally irradiated recipient mice (5000 LSKs were injected per recipient with 200,000 wild-type support bone marrow cells). c) Total numbers of GFP+ leukocytes, GFP+ B lymphocytes and GFP+ myeloid cells monitored 4, 8 and 10 weeks post-transplant in the peripheral blood of recipient mice. d) Representative flow cytometric analysis of peripheral blood for CD45.2+ GFP+ cells, with B220, IgM and IgD staining as indicated (n = 6–12 mice per genotype). Small horizontal lines indicate the mean. * P = <0.01 ** P = <0.001, *** P = <0.0001 in all panels.
