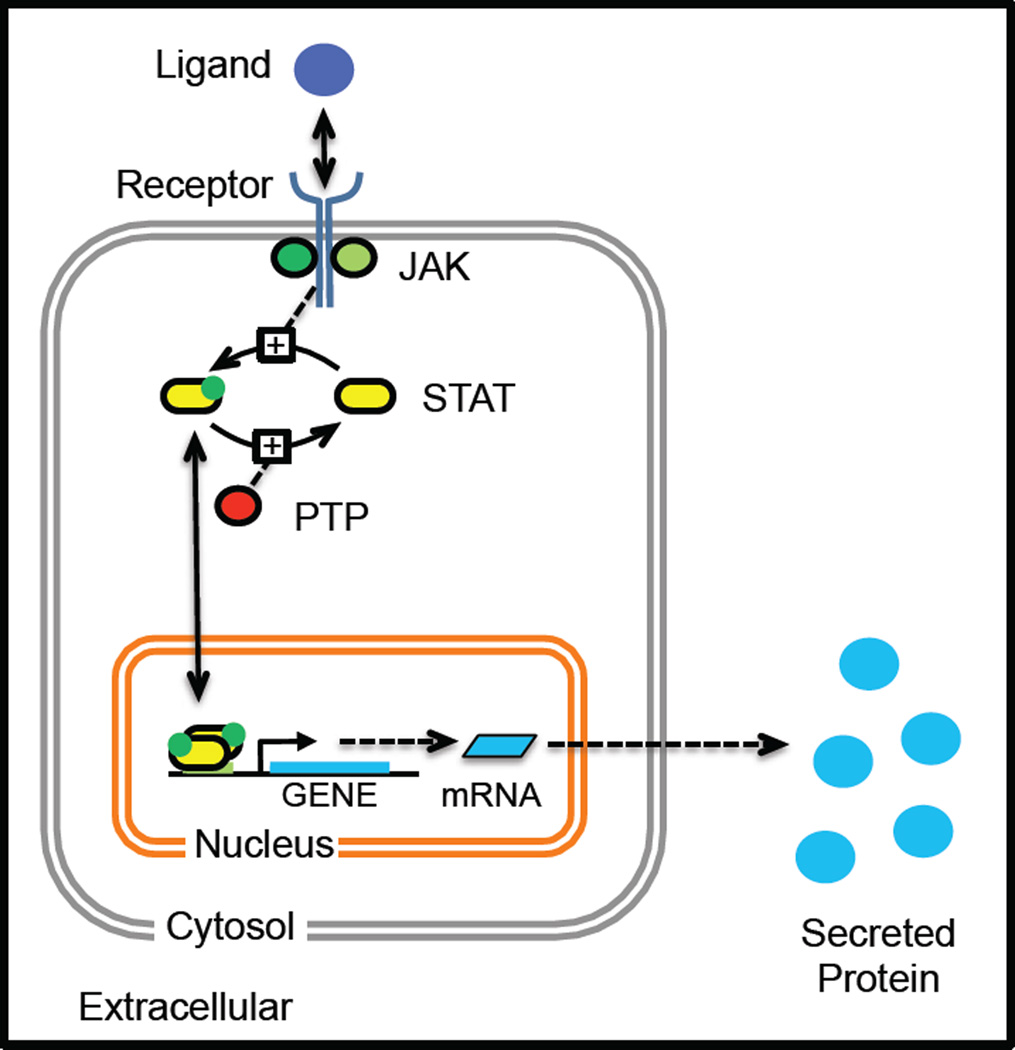
Figure 2. A cartoon illustrating the canonical JAK-STAT signaling pathway that initiates a cellular response, as depicted by secreted protein production, in response to stimulation with an extracellular ligand.
An extracellular soluble cue binds to a multi-protein complex that is comprised of transmembrane receptor proteins and associated Janus kinases. Upon ligand binding, the receptor changes conformation enabling the JAKs to phosphorylate STAT binding sites within the cytoplasmic tails of the receptors. The STAT proteins then associate with the activated receptor complex and sub-sequently become phosphorylated by the JAKs, as indicated by the green dot. The phosphorylated STAT proteins dimerize and migrate to the nucleus to initiate the transcription and translation of the corresponding STAT-responsive genes, which include cytokines that are released by the cell to help coordinate cellular response. STAT proteins become deactivated following dephosphorylation by a number of different phosphatases, including protein tyrosine phosphatases (PTP), that are present within the cell.