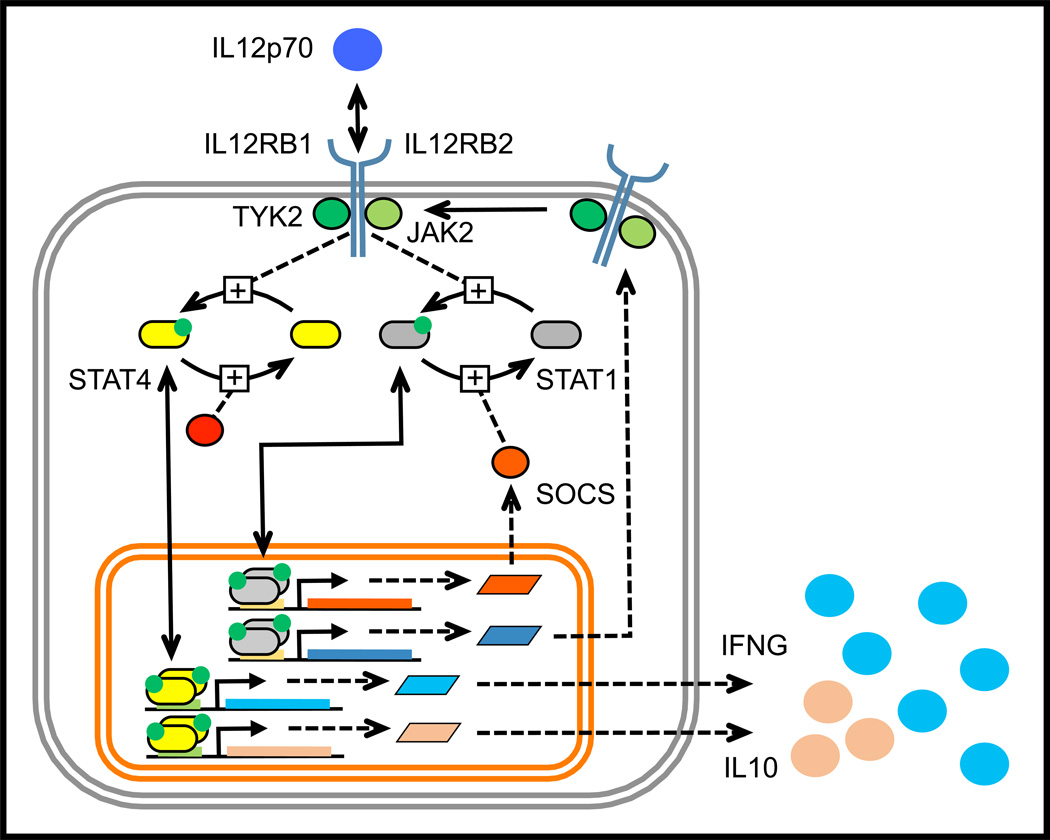
Figure 3. The emerging JAK-STAT pathways associated with IL12 signaling in type 1 T helper cells.
IL12 binds to a multi-protein receptor complex comprised of two transmembrane receptor proteins, IL12RB1 and IL12RB2, that are bound to the Janus kinases TYK2 and JAK2, respectively. Binding of the receptor complex by IL12 results in the phosphorylation of both STAT1 and STAT4. STAT1 plays a role in the expression of IL12RB2 while STAT4 promotes the transcription and translation of Interferon-γ (IFNG) and Interleukin-10 (IL10). While initially activated by IL12, STAT1 becomes dephosphorylated through an unclear mechanism that may involve the inducible expression of a phosphatase, like a Suppressor of Cytokine Signaling (SOCS). The intracellular transport of the receptor complex (i.e., receptor trafficking), dilution of proteins due to cell proliferation, and signaling pathways that connect IL12 stimulation to enhanced cell viability are some of the biological processes not depicted in this diagram.