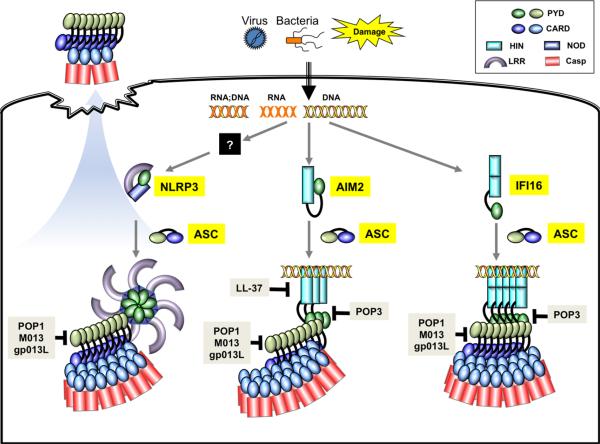
Fig. 1. Cartoon presentation of the ligand recognition, assembly, and regulation of nucleic acid-sensing AIM2, IFI16, and NLRP3 inflammasomes.
Even though NLRP3 has been found to be important for sensing of RNA:DNA hybrid and RNA, it is currently not clear whether it directly associates with these nucleic acids or functions through other binding partners. AIM2 resides in an autoinhibited state in the absence of dsDNA binding, whereas the conformation of the DNA-free IFI16 appears to be more extended. Similar to AIM2 and NLRC4, NLRP3 is presumed to be in an autoinhibited conformation in the resting state, in which its leucine-rich repeat (LRR) domain interacts with the NOD and PYD domains. Activation of AIM2 and NLRP3 leads to the formation of filaments involving the adapter ASC, whereas the IFI16–DNA complex has been shown to adopt filament structure in the absence of ASC. Upon pyroptotic cell death, large specks composed mostly of polymerized ASC are released into the extracellular environment to perpetuate inflammation. A number of host and microbe-encoded proteins or peptide have been shown to modulate inflammasome function through the regulation of ligand binding or PYD–PYD interactions. Keys to the individual domains are shown on the upper right corner.