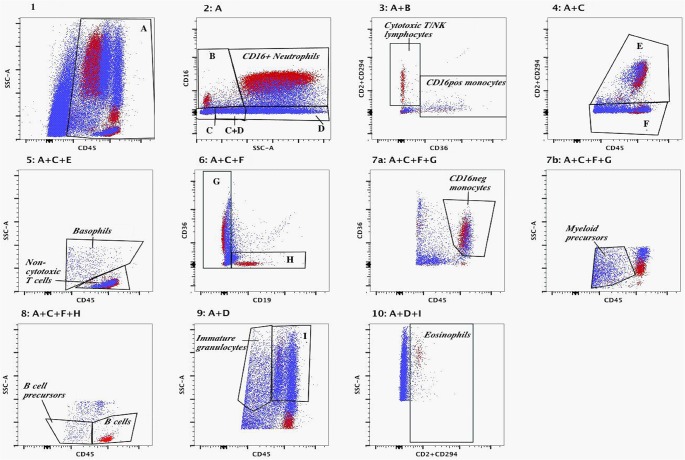
Fig 1. Overlay of gating strategy applied to peripheral adult blood (red) and to breast milk (blue), based on the method of Faucher et al.
The sequences of gates leading to each panel are shown above each panel. Panel 1: CD45 positive cells were gated as shown in A. Panel 2: CD45+ cells identified in Panel 1 were separated based on CD16 staining and side scatter properties, including a C16+/SSClow gate (B), and two overlapping gates of CD16- cells (C and D), and the CD16+ neutrophil population were identified. Panel 3: CD45+/CD16+/SSClow cells identified in Panel 2 gate B were separated into cytotoxic T and NK lymphocytes and CD16+ monocytes based on CD2/CD294 and CD36 staining properties. Panel 4: CD45+/CD16-/SSClow–intermediate cells (gate C) were separated based on CD2/CD294 positive (gate E) or negative (gate F) populations. Panel 5: From Panel 4, CD2 and/or CD294 positive cells (gate E) were gated into non-cytotoxic T cells or basophils using side scatter and CD45 staining properties. Panel 6: From Panel 4, CD2/CD294- cells (gate F) were gated into CD19+/CD36- cells (gate H) or CD19- cells (gate G). Panel 7a: Cells gated in G in Panel 6 with CD45high and CD36+ were identified as CD16 negative monocytes. Panel 7b: From Panel 6 gate G, CD45low cells with low side scatter were identified as myeloid precursor cells. Panel 8: From Panel 6, CD19 positive cells (gate H) were discriminated into B cells or B cell precursors based on CD45 staining though both populations displayed low side scatter properties. Panel 9: From Panel 2, CD16- cells with intermediate to high side scatter (gate D) and CD45low staining were identified as immature granulocytes, and those with intermediate to high CD45 staining properties and high side scatter were separated into gate I. Panel 10: From Panel 9, cells in gate I were identified by positive CD2/CD294 staining as eosinophils.