Fig. 9.
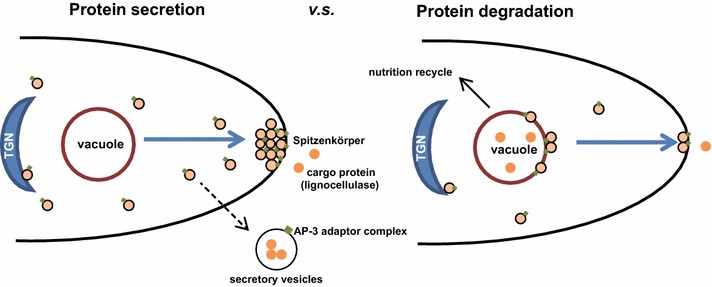
Schematic model illustrating a proposed mechanism for adaptor protein 3 (AP-3) complex-mediated lignocellulase fate decision in Neurospora crassa. When N. crassa cells are exposed to Avicel at an early stage (4 h), lignocellulases are synthesized and secreted into the extracellular matrix for nutrient acquisition. During this period, the AP-3 complex was observed to accumulate in the tip region—considered the main region for cellulolytic enzyme secretion. As high protein flux tends to temporarily lead to intracellular nitrogen source depletion, partial enzyme molecules at the logarithmic stage of fermentation (48 h) might suffer degradation so that the cell can obtain amino acids through nutrient recycling to maintain normal cell physiological activities such as somatic growth. At this time, the AP-3 complex was found to have re-located to vacuolar membranes, likely mediating delivery of cargo protein (lignocellulases) to secretory vesicles for degradation. Under this scenario, disruption of AP-3 complex function would lead to continued protein secretion, and the hypersecretion phenotype of ΔNcap3m can be reasonably explained.
