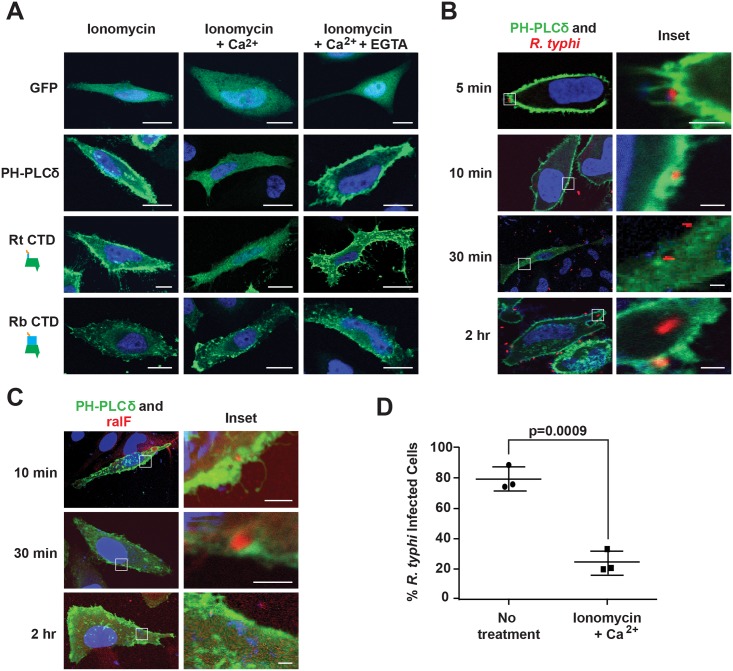
Fig 5. PI(4,5)P2 interacts with RalFRt and mediates R. typhi infection.
(A) RalFRtCTD co-localizes with PI(4,5)P2. HeLa cells transfected with pEYFP-C1 empty vector, GFP-C1-PLCδ-PH (a PI(4,5)P2 biosensor), EYFP–RalFRtCTD, or EYFP–RalFRbCTD were treated with 5 μM ionomycin alone, with Ca2+, or with Ca2+ and EGTA. Nuclei were stained with DAPI (blue). (Scale bar: 10 μm). (B) PI(4,5)P2 is recruited during R. typhi infection. HeLa cells transfected with GFP-C1-PLCδ-PH (green) were infected with R. typhi (MOI ~100:1) for indicated times. R. typhi was detected with rat anti-R. typhi serum and Alexa Fluor 594 anti-rat antibody (red). Nuclei were stained with DAPI (blue). Boxed regions are enlarged to show detail (inset). (Scale bar: 1 μm). (C) RalF localizes to PI(4,5)P2-enriched regions of the plasma membrane during R. typhi infection. HeLa cells transfected with GFP-C1-PLCδ-PH (green) were infected with R. typhi (MOI ~100:1) for indicated times. RalFRt was detected with rabbit anti-RalFRt and Alexa Fluor 594 anti-rabbit antibodies (red). Nuclei were stained with DAPI (blue). Boxed regions are enlarged to show detail (inset). (Scale bar: 1 μm). (D) Ionomycin and Ca2+ treatment decreases R. typhi infection. HeLa cells treated with 5 μM ionomycin and Ca2+ or no treatment were infected with R. typhi (MOI ~100:1) for 2 hrs. R. typhi was detected with rat anti-R. typhi serum and Alexa Fluor 488 anti-rat antibody. Cell membrane was stained with Alexa Fluor 594 wheat germ agglutinin. The number of infected host cells was counted, with percent infection of three independent experiments (100 host cells counted for each) plotted. Error bars represent mean ± SD (Student’s two-sided t-test).