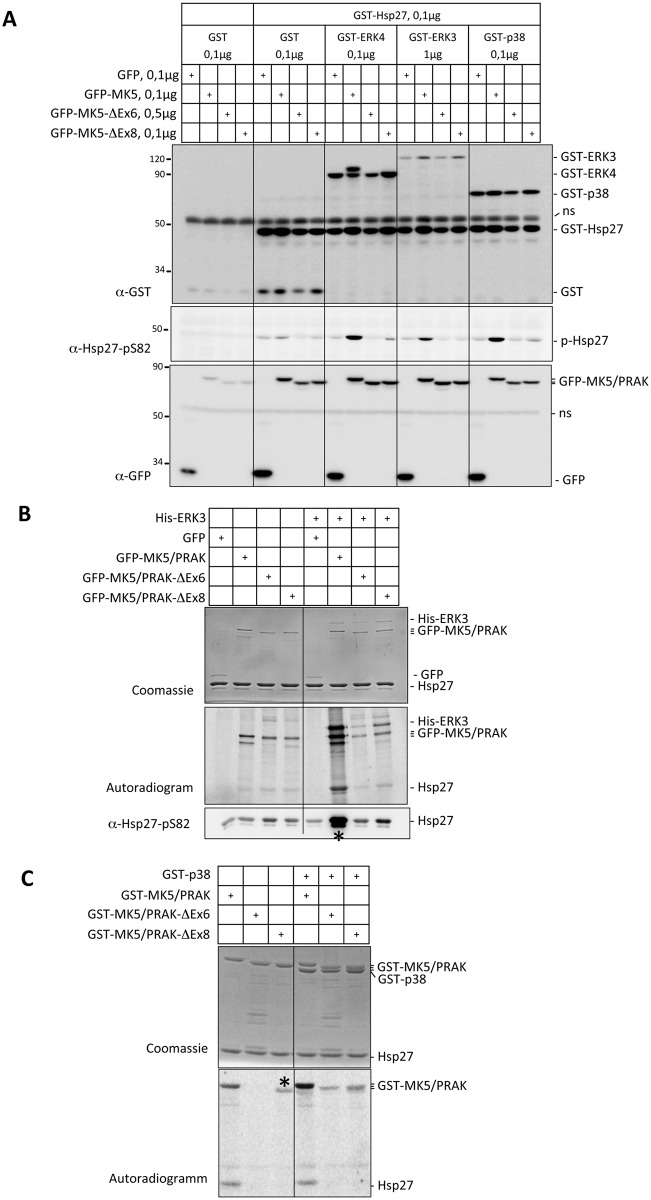
Fig 5. Kinase activity of wild type MK5/PRAK, MK5/PRAK-Δex6 and -Δex8.
A) HEK293 cells were transfected with the constructs indicated. The total amount of DNA per well was kept constant (1,6μg DNA per well in 12-well plate) by adding pcDNA-flag expression vector. 24 h post transfection cells were lysed in the plates with 1x Laemmli buffer. Lysates were analyzed by Western blotting using the indicated antibodies. A significant increase in phosphorylation of the substrate Hsp27 was only detected for WT MK5/PRAK in the presence of overexpressed p38 MAPK, ERK3 or ERK4. B) HEK293 cells were transfected with the expression constructs indicated. 24 h post transfection the cells were lysed and GFP-tagged MK5/PRAK or its exon 6 (Δex6) or exon 8 (Δex8) deletion mutants were immuno-precipitated by GFP nanobodies coupled to M270 epoxy beads. The beads were used in a kinase reaction with recombinant Hsp27 as a substrate. The reaction mixture was resolved by SDS-PAGE and Hsp27 phosphorylating activity was detected by phospho-imaging (autoradiogram) and Western blot against pS82-Hsp27. Increased Hsp27-kinase activity is only detected for WT MK5/PRAK (asterisk). C) In vitro MK5/PRAK kinase assay with Hsp27 as substrates. GST–p38 was used to activate MK5/PRAK in vitro. While Hsp27-kinase activity is only detected for WT MK5/PRAK, auto-phosphorylating activity in the absence of p38 MAPK (left panel) is detected for both WT MK5/PRAK and Δex8 (asterisk).