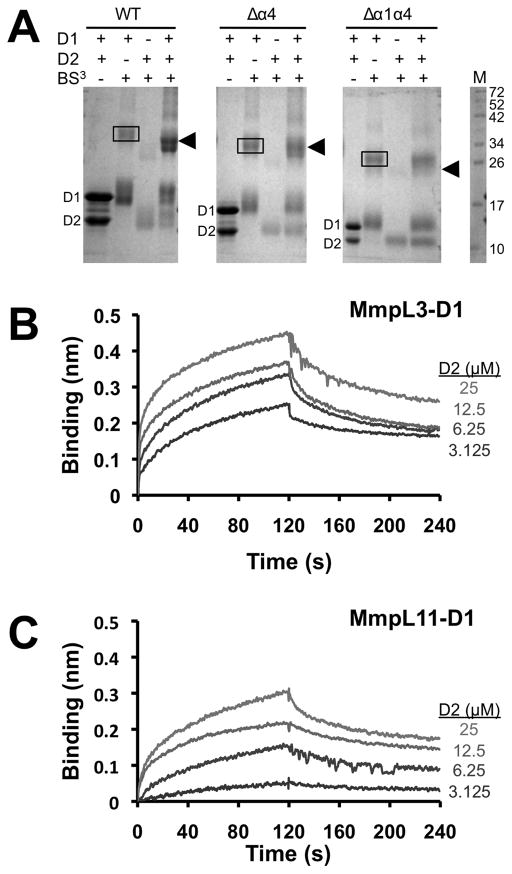
Figure 4.
MmpL3 and MmpL11 D1 and D2 domains interact. (A) SDS-PAGE of MmpL3 D1 and D2 domains and their respective truncated constructs (Δα4 and Δα1α4) in the presence of BS3, suggesting that α1 helix is essential for heterodomain interaction. In all instances, the MmpL3-D1 homodimer (38.3 kDa for WT) is boxed whereas arrowheads identify the MmpL3-D1-D2 heterodimer (34.9 kDa for WT). Notably, for the Δα1α4 constructs, the heterodimer is absent. Biolayer interferometry experiments to assess interactions between (B) MmpL3 D1 (biotinylated) and D2 domains, and (C) MmpL11 D1 (biotinylated) and D2 domains. All reactions were performed at 25 °C in 20 mM sodium phosphate pH 7.4 and 150 mM NaCl. Immobilized biotinylated D1 domains were exposed to different concentrations (25 – 3.125 μM) of D2 domains, where interaction (association and dissociation) is assessed by a wavelength shift (nm).