Figure 2.
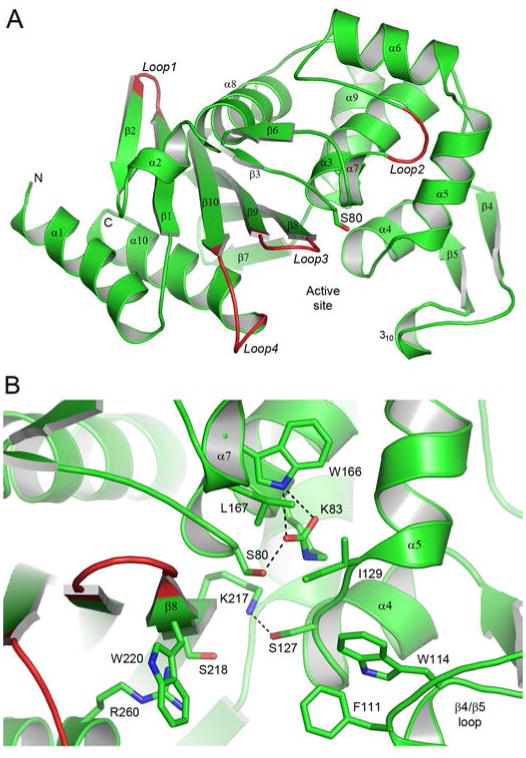
The OXA-51 structure. (A) Ribbon representation of the A. baumannii OXA-51 β-lactamase showing the secondary structure labelling used. The location of the active site is indicated by the side chain of the catalytic serine, Ser80. Four loops (loops 1 - 4) which show the largest structural variation between OXA-51 and the other OXA enzymes, whose structures are known, are also indicated. (B) Close-up view of the OXA-51 active site showing some of the conserved and partially-conserved residues which are important for substrate binding and/or the enzyme mechanism.
