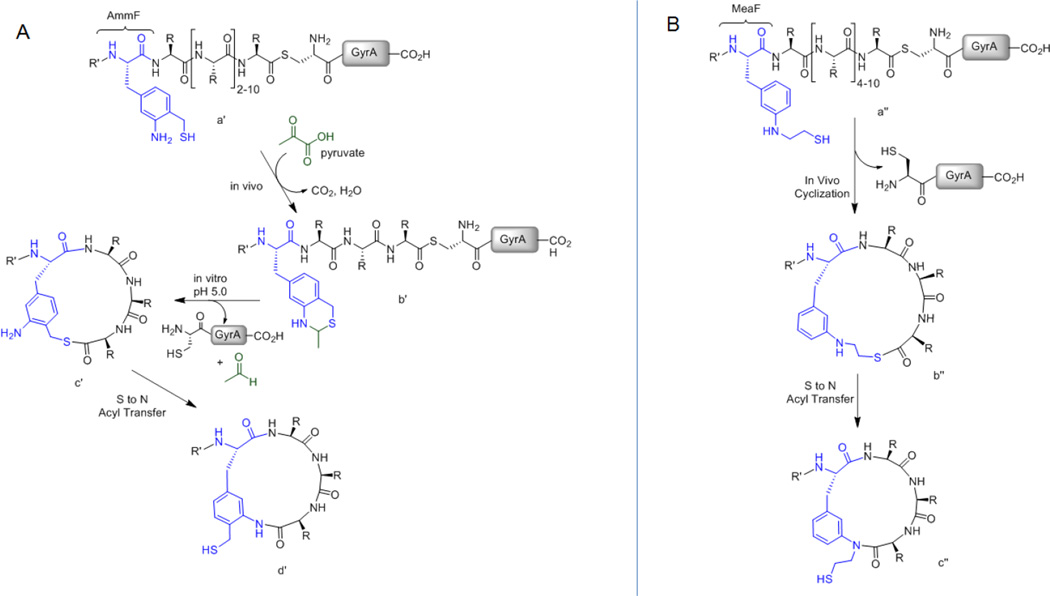
Figure 3.
Precursor polypeptides, reaction intermediates and final macrocyclic peptide products for (A) AmmF-mediated and (B) MeaF-mediated peptide cyclization. (A) Expression of the AmmF-containing precursor protein (a′) in E. coli results in the formation of the benzothiazine adduct (b′), which upon pH-induced deprotection in vitro leads to the target macrocyclic peptide (d′). (B) Expression of the MeaF-containing precursor protein (a″) in E. coli results in spontaneous, post-translational peptide cyclization to give the thiolactone intermediate (b″) and then the final macrolactam product (c″) via an S→N acyl shift rearrangement.