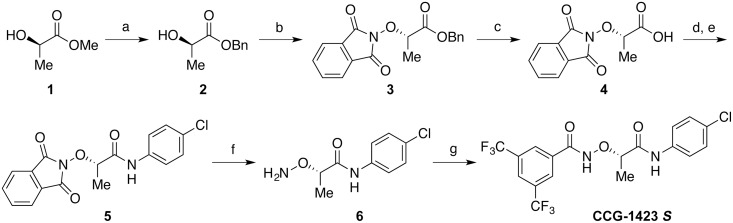
Fig 2. Synthesis of optically active CCG-1423 S.
Reagents and conditions: (a) benzyl alcohol, iron(III) acetylacetonate, Na2CO3, heptane, reflux (–H2O), 12 h, 87%; (b) N-hydroxyphthalimide, dimethyl azodicarboxylate, PPh3, THF, RT, 1 h, 63%; (c) H2, Pd-C, MeOH, RT, 2 h, 94%; (d) (COCl)2, N,N-dimethylformamide, CH2Cl2, RT, 1 h; (e) 4-chloroaniline, CH2Cl2, 0°C, 2 h, 66% (2 steps); (f) NH2NH2-H2O, MeOH, RT, 5 min, 52%; (g) 3,5-bis(trifluoromethyl)benzoic acid, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, 4-dimethylaminopyridine, CH2Cl2, RT, 1 h, 79%.