Abstract
Myeloproliferative neoplasm (MPN) transformed to acute myeloid leukemia (MPN-AML), MPN in accelerated phase (MPN-AP), and high-risk primary myelofibrosis (PMF) are associated with a poor response to therapy and very short survival. Several reports have suggested clinical activity of hypomethylating agents in these patients. We conducted a retrospective study of 21 patients with MPN-AML, 13 with MPN-AP and 11 with DIPSS-plus high-risk PMF treated with decitabine at our institution over the last 7 years and evaluated their clinical outcomes. Six patients (29%) with MPN-AML responded to decitabine (3 CR, 2 CRi, and 1 PR); median response duration was 7 months. The median overall survival (OS) was significantly higher in those who responded (10.5 vs 4 months). Among patients with MPN-AP, 8 patients (62%) benefited; median response duration was 6.5 months. The median OS was 11.8 months in responders vs 4.7 months in non-responders. Among patients with DIPSS-plus high-risk PMF, 9 (82%) benefited; median response duration was 9 months. The median OS was 32 months in responders vs 16.3 months in non-responders. Decitabine is a viable therapeutic option for patients with MPN-AML, MP-AP and high-risk PMF. Prospective clinical studies combining decitabine with other clinically active agents are needed to improve overall outcome.
Keywords: myeloproliferative neoplasm, AML, accelerated phase, myelofibrosis, decitabine
1. Introduction
Philadelphia (Ph) chromosome–negative myeloproliferative neoplasms in the blastic/acute myeloid leukemia (MPN-AML) or accelerated (MPN-AP) phase and those defined as high-risk primary myelofibrosis (PMF) by the Dynamic International Prognostic Scoring System-plus (DIPSS-plus) have been associated with a poor response to therapy and severely shortened survival.[1–3] For patients with high-risk PMF a number of treatment modalities have been explored, including proteasome inhibitors, immunomodulatory agents (thalidomide, lenalidomide and pomalidomide), farnesyl transferase inhibitors, and others, with limited or no benefit.[4–8] The advent of ruxolitinib, a JAK inhibitor, has greatly improved the signs and symptoms associated with PMF, including control of constitutional symptoms, reduction in splenomegaly, and improvement in performance status and quality of life.[9, 10] Patients with high-risk PMF who have a good response to ruxolitinib may live for several years longer than expected.[11] However, some patients have disease that is refractory or doesn’t respond satisfactorily to ruxolitinib, while others may lose their response, warranting new treatment strategies. The current therapeutic strategies for patients with MPN-AML and MPN-AP rarely offer more than palliative benefit.[12] Transformation of Ph-negative MPNs into the blastic phase (MPN-AML) occurs at a rate of 10–20% after a follow up of 15–20 yrs.[13] Patients with MPN-AML respond poorly to chemotherapy and have a median survival of 3–5 months.[2, 14, 15] Stem cell transplant (SCT) has proven to be the only successful treatment modality among long-term survivors of these 3 groups. However, in the majority of patients SCT it is not a feasible option due to advance age, co-morbidities and poor performance status. Moreover, patients with MPN-AP and MNP-AML, initially require intensive chemotherapy to reduce the disease burden to become eligible for SCT. Consequently, overall fewer than 10% of patients undergo SCT.[14]
Several studies have hypothesized that PMF develops after the acquisition of gene mutations and changes in epigenetic modification, resulting in silencing of genes that control cell proliferation, differentiation and apoptosis. Vannucchi and colleagues analyzed a number of mutations in cohort of 879 patients with MF to determine their prognostic value. ASXL1, SRSF2 and EZH2 were found to be independently associated with poor survival. However, only ASXL1 had prognostic significance independent of DIPSS-plus model.[16] The authors also demonstrated that MF patients harboring IDH1/2, SRSF2 and ASXL1 mutations had shortened leukemia free survival. Furthermore, epigenetic modifications such as DNA hypermethylation of the p15INK4B and p16INK4A genes located on chromosome 9p21 and retinoic acid receptor β have been reported in the pathogenesis of MPN-AP and MPN-AML.[17, 18] One striking feature of PMF is the abnormal trafficking of CD34+ cells. Patients with PMF have 20–30 times more circulating CD34+ cells than those with polycythemia vera and essential thrombocythemia, and increased circulating CD34+ cells correlates with worse outcomes.[19] In a mouse model of MF, the abnormal trafficking of PMF CD34+ cells can be reversed by chromatin-modifying agents, such as hypomethylating agents, extending earlier observations from an ex vivo study. [20, 21] Nischal et al[22] found differential genome-wide methylation patterns in polycythemia vera (PV), essential thrombocythemia (ET) and PMF samples compared with healthy controls,. Interestingly, all MPN-derived cells with aberrant DNA methylation were sensitive to hypomethylating agent (decitabine). PMF with ASXL1 mutations had relatively more DNA methylation and were the more sensitive to decitabine than PMF without these ASXL1 mutations, whereas the JAK2 mutation did not alter the DNA methylation pattern. These results provide a scientific rationale for the development of epigenetic approaches in patients with advanced PMF, MPN-AP and MPN-AML.[23, 24] Both azacitidine and decitabine have been approved by the Food and Drug Administration for the treatment of patients with myelodysplastic syndrome (MDS).[25, 26] A few small studies have evaluated the role of hypomethylating agents in patients with PMF and MPN-AML.[27–29] Here we present a retrospective analysis of data from group of patients with MPN-AML, MPN-AP and DIPSS-plus high-risk PMF treated with decitabine at our institution.
2. Patients and Methods
We retrospectively reviewed the charts of all patients with MPN treated at The University of Texas MD Anderson Cancer Center during the past 7 years. We identified 21 patients with MPN-AML, 13 with MPN-AP and 11 with DIPSS-plus high-risk PMF who were treated with decitabine, either alone or in combination with targeted therapies, including gemtuzumab ozogamicin (GO; a CD33 antibody-drug conjugate) and ruxolitinib (a JAK inhibitor). MPN-AML was defined as patients with MPN that had transformed to AML according to WHO 2008 criteria.[30] MPN-AP was defined as MPN with 10%–19% blasts in the peripheral blood or bone marrow (BM). DIPSS-plus high-risk PMF was defined according to the DIPSS-plus scoring system for PMF.[3] Responses in MPN-AML were defined according to published recommendations from the post-MPN-AML consortium.[31] Complete remission (CR) indicates a complete remission of leukemia with residual MPN features such as splenomegaly and MPN-associated cytogenetic and molecular abnormalities; partial response (PR) is defined as decrease in leukemic burden but with residual blasts in the bone marrow or peripheral blood; stable disease (SD) means failure to achieve partial response and no evidence of PD in either MPN or leukemia. Responses in MPN-AP and DIPSS-plus high-risk PMF were defined according to the revised IWG-MRT and ELN consensus report.[32] We also appreciated through the outcome analysis, a group of patients with SD achieving clinical benefit (e.g. significant decrease in blast percentage or leukocytosis, or increase in blood counts), not recognized as a response category or not satisfying a response definition, who experienced prolonged good control of the disease; we called this group “SD with clinical benefit”. Response duration was defined as the time between achieving a response and disease progression, next therapy (including transplant), or last follow-up/death. Patients received decitabine 20 mg/m2 intravenously for 5 days every 28 days. In some patients, the decitabine dose was adjusted after cycle 1, according to patient tolerance. Some patients also received GO on day 5 of cycle 1 at a dose of 3 mg/m2, or 25 mg ruxolitinib orally twice daily continuously. After cycle 1, the ruxolitinib dose was titrated according to patient tolerance. All patients with a response continued on treatment until disease progression or death. Patients who were eligible went on to SCT after achieving a response to decitabine and based on donor availability.
2.1. Statistical analysis
Categorical data were tabulated by frequency and percentage; continuous variables were summarized using descriptive statistics (median, range). Overall survival (OS) was defined from the date of presentation at our institution to the date of death/last follow-up for patients with high-risk PMF. For patients with MPN-AML or MPN-AP, OS was defined from the date of transformation to AML or AP, respectively, and the date of death/last follow-up. The median overall survival was estimated by the Kaplan-Meier method, and median survival times of different cohorts were compared using the log-rank test.
3. Results
3.1. Patient characteristics
Baseline characteristics of patients are summarized in Table 1. Twelve (57%) patients with MPN-AML, 12 (92%) with MPN-AP, and 5 (45%) with DIPSS-plus high-risk PMF received decitabine alone. None of the patients died during the first 4 weeks of therapy with decitabine whether alone or in combination with GO or ruxolitinib.
Table 1.
Baseline characteristics
| Variables | Median [range] or n (%) | ||
|---|---|---|---|
| MPN-AML (n=21) | MPN-AP (n= 13) | High risk DIPSS plus PMF (n= 11) | |
| Age in years | 64 [45–82] | 63 [50–81] | 67 [55–77] |
| Gender | |||
| Male | 16 (76) | 8 (62) | 8 (73) |
| Female | 5 (24) | 5 (38) | 3 (27) |
| Initial MPN | |||
| ET | 4 (19) | 2 (15) | 0 (0) |
| PV | 5 (24) | 5 (39) | 0 (0) |
| PMF | 10 (48) | 6 (46) | 11 (100) |
| MPNu | 2 (10) | 0 (0) | 0 (0) |
| WBC (109/L) | 8 [1–55] | 11.6 [1–50] | 41.5 [2–140] |
| Hb (g/dl) | 9 [8.3–11.5] | 9.5 [6–11.7] | 9.2 [7.7–11.7] |
| Plt (109/L) | 39 [3–1912] | 73 [14–267] | 69 [9–860] |
| Bone marrow blasts (%) | 31 [21–81] | 14 [10–17] | 2 [0–9] |
| JAK2V617F mutation | 11 (52) | 8 (62) | 7 (64) |
| FLT3 ITDa | 1 (5) | 1 (8) | 0 |
| RASb | 0 | 0 | 2 (25) |
| IDH1/2c | 0 | 2 (28) | 0 |
| DNMT3Ad | 0 | 0 | 0 |
| ASXL1e | 0 | 0 | 0 |
| NPM-1f | 0 | 1 (14) | 0 |
| EZH2g | 0 | 0 | 0 |
| TET2h | 0 | 0 | 0 |
| SRSF2i | 0 | 0 | 0 |
| Cytogenetics[1, 3] | |||
| Unfavorable* | 12 (57) | 6 (46) | 2 (18) |
| No. of prior therapies for MPN | 1 [0–4] | 2 [1–5] | 1 [0–4] |
| Time to Decitabine (months) | 8 [0.5–241] | 65 [0–389] | 19 [3–195] |
| Decitabine as a: | |||
| 1st line of therapy | 12 (57) | 1 (8) | 2 (18) |
| 2nd line of therapy | 8 (38) | 2 (15) | 4 (36) |
| 3rd or greater line of therapy | 1 (5) | 10 (77) | 5 (45) |
| Decitabine alone | 12 (57) | 12 (92) | 5 (45) |
| Decitabine + gemtuzumab ozogamicin | 7 (33) | 0 (0) | 5 (45) |
| Decitabine + ruxolitinib | 2 (10) | 1 (8) | 1 (10) |
| No. decitabine cycles | 2 [1–15] | 2 [1–37] | 3 [1–8] |
| Mortality after ≤ 4 weeks of therapy | 0 (0) | 0 (0) | 0 (0) |
DIPSS; dynamic international prognostic scoring system, MPN; myeloproliferative neoplasm, ET; essential thrombocytosis, PV; polycythemia vera, PMF; primary myelofibrosis, AML; acute myeloid leukemia, AP; accelerated phase, WBC; white blood cell, Hb; hemoglobin, Plt; platelet.
Unfavorable: (complex, +8, -7/7q-, i (17q), -5/5q-, 12p-, inv (3), or 11q23).
18 MPN-AML, 12 MPN-AP and 8 PMF patients were evaluated for FLT3 ITD;
12 MPN-AML, 8 MPN-AP and 8 PMF were evaluated for RAS;
12 MPN-AML, 7 MPN-AP and 6 PMF evaluated for IDH1/2;
5 MPN-AML, 5 MPN-AP and 6 PMF were evaluated for ASXL1, DNMT3A, EZH2, TET2 and SRSF2;
10 MPN-AML, 7 MPN-AP and 7 PMF patients were evaluated for NPM-1 mutation.
3.2. Responses
Responses are summarized in Table 2. Six (29%) patients with MPN-AML had responses: 3 CR, 2 CRi and 1 PR. Among the patients who achieved a response, 1 received decitabine along with GO and the rest received decitabine alone. The median number of cycles to response was 2 (range, 1–13) and the median response duration was 7 months (range, 2–24+). Interestingly, 2 patients who achieved a CR received decitabine as a second-line therapy after failing anthracycline plus cytarabine-based chemotherapy. Among non-responders, 10 (48%) patients died due to disease progression, 3 (14%) died due to sepsis, one is alive with stable disease (SD) on different therapy, and one patient died 2 months after SCT following a response to a different therapy. One patient who achieved a CR with decitabine also died 2 months after matched unrelated donor SCT.
Table 2.
Responses in MPN-AML, MPN-AP and DIPSS-plus high-risk PMF
| Responses in MPN-AML (n= 21) | Median [range] or n (%) |
|---|---|
| Overall response | 6 (29) |
| CR | 3 (14) |
| CRi | 2 (9) |
| PR | 1 (5) |
| Median number of cycles to response | 2 (1–13) |
| Response duration (months) | 7 (2–24+)* |
| Responses in MPN-AP (n= 13) | |
| Overall response | 8 (61) |
| CI in Hb & Plt | 1 (8) |
| SD with clinical benefit ab | 7 (54) |
| Median number of cycles to response | 2 (1–6) |
| Response duration (months). | 6.5 (2–14+)b |
| Responses in DIPSS-plus high-risk PMF (n= 11) | |
| Overall response | 9 (82) |
| CI platelet | 1 (9) |
| CI spleen | 1 (9) |
| SD with clinical benefit | 7 (64) |
| Median number of cycles to response | 3 (1–4) |
| Response duration (months) | 9 (1–23+)* |
1 patient in CR went for SCT after 2 months of achieving response
SD with clinical benefit (improvement in bone marrow and peripheral blood blast %, cytopenias and leukocytosis)
4 patients with SD went to SCT
2 patients with SD with clinical benefit (improvement in bone marrow and peripheral blood blast %, cytopenias and leukocytosis) went to SCT.
CR; complete remission, CRi; complete remission with incomplete count recovery, PR; partial remission, CI; clinical improvement, Hb; hemoglobin, Plt; platelet, SCT; stem cell transplant.
In the MPN-AP group, 1 (8%) patient had clinical improvement (CI) in Hb levels and platelet counts. Seven (54%) patients had SD but with improved blood cell counts or reduced percentage of BM blasts (not achieving official response criteria). The median number of decitabine cycles to response were 2 (range, 1–6) and the median response duration was 6.5 months (range, 1.8–14+). Four (31%) patients achieving SD with clinical benefit had SCT. Eventually, transformation to AML was seen in 3 of 8 responders and 2 of 5 non-responders. The median time to leukemia transformation from MPN-AP was 3.7 months (range, 2–5) in responders and 2.4 months (range, 1–3.6) in non-responders. For patients with MPN-AML or MPN-AP, the MPN diagnosis before transformation/progression was not correlated with response to decitabine: patients with an initial diagnosis of ET, PV, or PMF had response rates of 33%, 50%, and 44%, respectively (not statistically different).
In the DIPSS-plus high-risk PMF group, 9 (82%) patients benefited from decitabine, but none achieved a response according to IWG-MRT criteria for CR or PR. One (9%) patient had CI in platelet counts, 1 (9%) had CI in spleen size, and 7 (64%) had stable disease with clinical benefit (improvements in blood counts and BM blast %). Among 9 responders, 5 (55%) received decitabine in combination with GO. The median number of decitabine cycles for response were 3 (range, 1–4), and the median response duration was 9 months (range, 1–23+). Three (27%) patients had SCT, including 2 who achieved SD with clinical benefit. Interestingly, all patients who achieved SD with clinical benefit were leukemia free as of the date of last follow-up, while 2 patients who did not respond progressed to AML and died.
Genetic analysis of selected mutations was available for a subset of patients described in this report (Table 1). We found no correlation between any of the mutations and response to decitabine (data not shown).
3.3. Survival
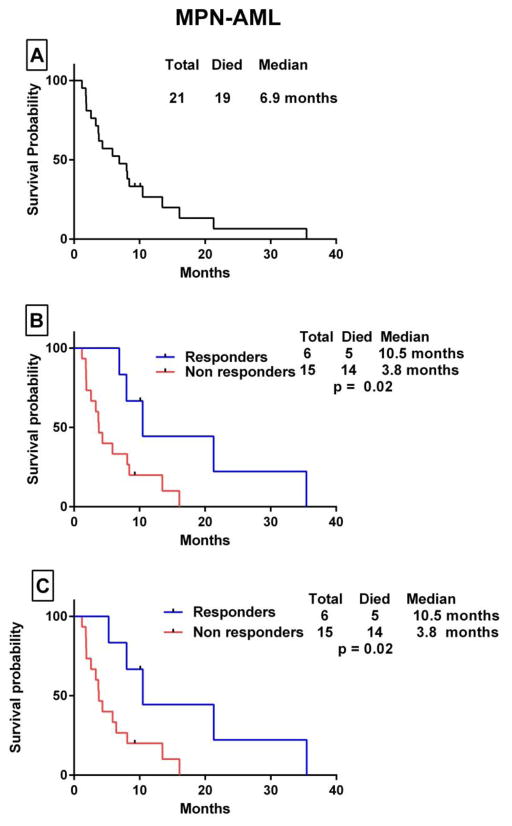
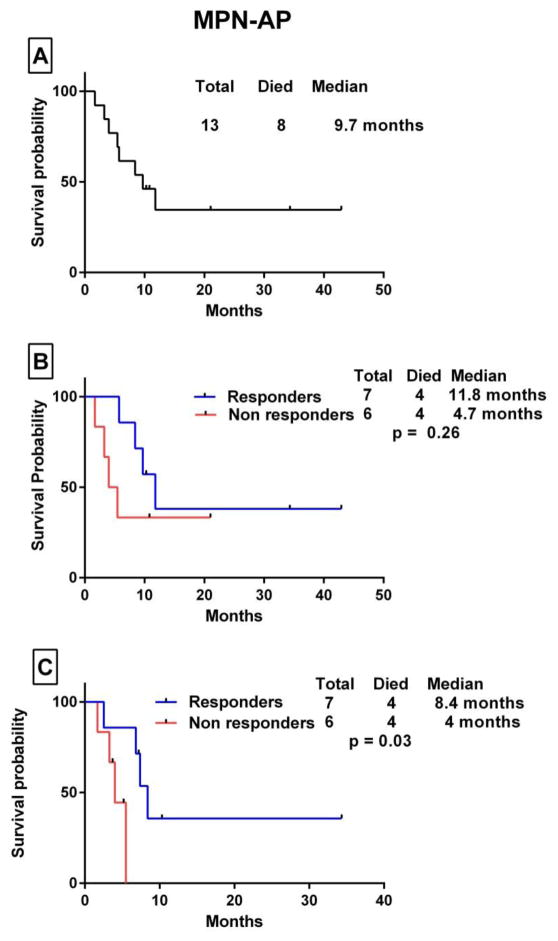
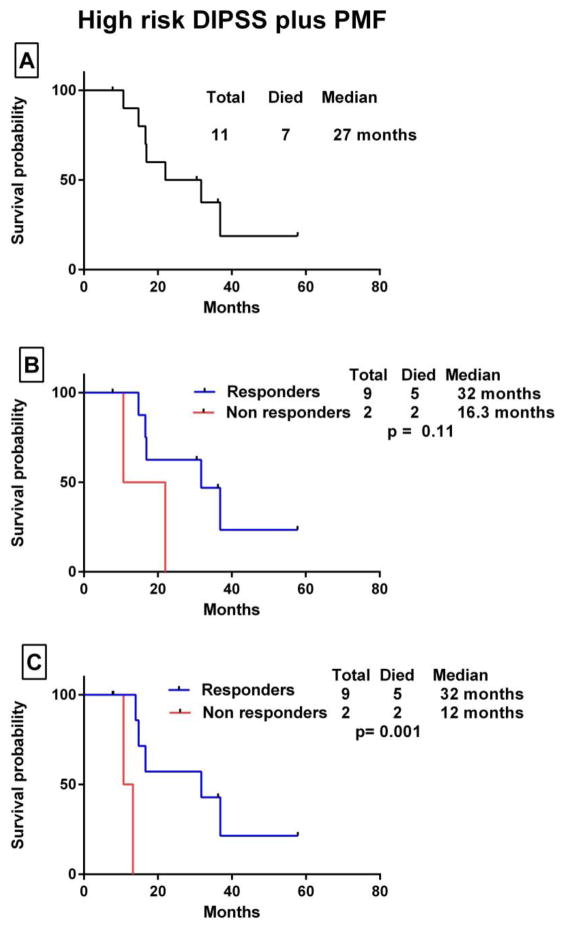
The median OS in patients with MPN-AML from the time of transformation to AML to death/last follow-up was 6.9 months. The median OS was 10.5 months in responders vs 4 months in non-responders (p= 0.02). When patients were censored at the time of SCT, the median OS was 10.5 months in patients who had a response and 3.8 months in those who did not (p= 0.02) (Fig. 1). Among patients with MPN-AP, the median OS from the time of progression to accelerated phase to death/last follow-up was 9.7 months. For responders, the OS was 11.8 months compared with 4.7 months for non-responders (p=0.26). When patients were censored at the time of SCT, OS was 8.4 vs 4 months, respectively (p= 0.03) (Fig. 2). The median OS in patients with high-risk DIPSS PMF was 27 months. The median OS was 32 months in responders vs 16.3 months in non-responders (p=0.11). When patients were censored at the time of SCT, the median OS was 32 months vs 12 months, respectively (p= 0.001) (Fig. 3).
Figure 1.
Kaplan Meier survival analysis of patients with MPN-AML. (A) Overall survival after transformation to leukemia. (B) Comparison of OS for patients who had a response to decitabine-based therapy (responders) vs those who did not (non responders) by the log-rank test. (C) OS survival comparison with patients censored at the time of stem cell transplant.
Figure 2.
Kaplan Meier survival analysis of patients with MPN-AP. (A) Overall survival after progression to the accelerated phase. (B) Comparison of OS for patients who patients who derived some clinical benefit (responders) vs those who did not (non responders) by the log-rank test. (C) OS survival comparison with patients censored at the time of stem cell transplant.
Figure 3.
Kaplan Meier survival analysis of patients with DIPSS-plus high-risk PMF. (A) Overall survival from the date of presentation. (B) Comparison of OS for patients who patients who derived some clinical benefit (responders) vs those who did not (non responders) by the log-rank test. (C) OS survival comparison with patients censored at the time of stem cell transplant.
Because decitabine has been reported to improve survival more than conventional chemotherapy in a small group of patients with MF transformed to AML, [27] we compared OS of subset of MPN-AML patients treated with decitabine as first-line therapy (n= 12; part of this report) to that of patients treated with intensive induction chemotherapy (high dose cytarabine [1000mg/m2/dose/day] based regimens) without SCT consolidation as first-line therapy. These patients were from a historical MPN-AML patient cohort treated at our institution. The median OS in 35 MPN-AML patients treated with intensive induction chemotherapy was 7.6 months compared with 6.9 months in those treated with decitabine (p= 0.4).
3.4. Stem cell transplant
Overall, 7 (15%) patients who had a response to decitabine had SCT (1 patient with MPN-AML, 4 with MPN-AP and 2 with high-risk PMF). Three patients had matched unrelated donors, 2 had matched related donors and 2 had haploidentical donors. Five patients died after SCT, 4 of them within the first 100 days after SCT. Two patients died due sepsis/multi-organ failure, 2 due to relapse/progressive disease and 1 due to grade IV graft versus host disease (Table 3).
Table 3.
Characteristics of patients treated with SCT after deriving clinical benefit from decitabine therapy
| Diagnosis | Age (yrs)/Gender | Cytogenetics | Response | Donor | Prior therapies before Decitabine | Survival from SCT (months) | Outcome |
|---|---|---|---|---|---|---|---|
| MPN→ AML | 70/M | Complex | CR | MUD | Hydrea, anagrelide, ruxolitinib, idarubicin + cytarabine | 1.6 | Died due to pneumonia/sepsis associated with relapse disease. |
| PMF→ AP | 69/F | 46,XX,der(15)t(1;15) (q12;q26.3) | SD with clinical benefit (decrease in PB and BM blast and resolution of leukocytosis) | MRD | ESA, G-CSF, revlimid, thalidomide, and imatinib | 40+ | Had GI and vaginal mucosa GVHD. After 35 months lost donor engraftment, restarted on Decitabine 10mg/m2 (5 days/monthly). Alive |
| PMF→ AP | 58/M | Diploid | CI (Hb and Plt) | MUD | Pomalidomide | 3 | Relapse as AML 2 months after SCT. Died due to progressive disease. |
| PV→AP | 63/F | 46,XX,del(20) (q11.2q13.3) | SD with clinical benefit (decrease in PB blast, improvement in Plt and resolution of leukocytosis) | Haplo | Hydrea, cytarabine, prednisone and ruxolitinib. | 3+ | Died due to GI GVHD and sepsis/MOF. |
| PV→AP | 54/M | Complex | SD with clinical benefit (disappearance of PB blast, improvement in Plt and resolution of leukocytosis | Haplo | Hydrea | 4+ | Relapse as AML 3 months after SCT. Died due to PD. |
| High risk PMF | 60/F | Diploid | CI (platelet) | MUD | Hydrea | 3 | Died due to grade 4 GVHD; GI and skin and sepsis. |
| High risk PMF | 55/M | 46,XY,del(11) (p13p15) | SD with clinical benefit (improvement in PB and BM blast) | MRD | Ruxolitinib | 22+ | Had grade 4 GI GVHD. In CR and alive. |
CR; complete remission, SD; stable disease, CI; clinical improvement, BM; bone marrow, PB; peripheral blood, Haplo; haploidentical stem cell transplant, MUD; match unrelated donor stem cell transplant, MRD; match related donor transplant, GVHD; graft versus host disease, MOF; multi organ failure, complex; ≥ 3 chromosomal abnormalities.
4. Discussion
Experience at our institution suggests that decitabine is a viable therapeutic option for patients with MPN-AML, MPN-AP and DIPSS-plus high-risk PMF. Decitabine is a safe therapy that can be delivered in an outpatient setting with satisfactory control of the disease in a good proportion of patients. While CR and PR are relatively rare achievements, our retrospective analysis suggests that benefits not readily measured (we identify these patients as having SD with clinical benefit) are important and may prolong survival of these patients with otherwise very poor outcome. Despite the small groups of patients studied, one may appreciate that OS of responders compared with non-responders was increased by almost 100% in all 3 subgroups.
The prognosis of MPN-AML patients remains poor despite major therapeutic advances, with median survival times of 3–5 months even after implementation of intensive AML-type chemotherapy.[2, 14, 15] Hypomethylating agents are less toxic and better tolerated than standard intensive chemotherapy regimens, especially in an elderly population, and can be delivered in outpatient setting. In a series of 54 patients with MPN that had transformed to AML or MDS who were treated with azacytidine as a first-line therapy, 52% achieved a response (CR/CRi/PR/HI) according to 2006 IWG criteria;[33] the median OS was 11 months.[28] In a separate report, decitabine has also improved survival in a small group of patients with MF transformed to AML, better than reported with conventional chemotherapy.[27] To follow on these observations, we compared the OS in subset of MPN-AML patients treated with decitabine as first-line therapy (n= 12; part of this report) to that of patients treated with intensive induction chemotherapy without SCT consolidation as first-line therapy. We found no significant difference in the OS between MPN-AML patients treated with intensive induction chemotherapy and those treated with decitabine (p= 0.4). However, the median age of the two groups differed significantly (one of many possible biases in comparing the two groups): 66 years (range, 40–80) for the intensive therapy group vs. 74 years (range, 55–82) for the decitabine group. In addition, it would have been unlikely that the 12 patients treated with decitabine would have received intensive chemotherapy, if not for other reasons, just based on their older age.
Treatment with ruxolitinib, a JAK inhibitor, has significantly improved the outcome of patients with intermediate-2 and high risk MF.[9, 10, 34] Its use in patients with MPN-AP has not been specifically studied; however, its activity in patients with MPN-AML is very limited.[35] Some investigators have already treated patients with a combination of ruxolitinib and hypomethylating agent with the aim of providing patients with both the anti-proliferative and anti-inflammatory potential of JAK inhibitors and the bone marrow-modifying effect of hypomethylating agents. Tabarroki and colleagues[36] reported on three MF patients treated with decitabine and ruxolitinib who derived clinical benefit, including reduction in spleen size, decrease in red blood cell transfusions, and a decrease in circulating peripheral blasts. Several trials are underway evaluating the efficacy of azacytidine plus ruxolitinib (ClinicalTrials.gov, NCT01787487) and decitabine plus ruxolitinib (ClinicalTrials.gov, NCT02076191) in patients with MPN-AP and MPN-AML. Because of the small numbers, we cannot make any conclusion about whether the addition of ruxolitinib or GO to decitabine in some patients had any impact on the patients’ responses or outcomes. Other epigenetic modifiers, such as histone deacetylase inhibitors, e.g. panobinostat, that have shown activity as a single agent in patients with MF, [37] are now being tested in combination with ruxolitinib (ClinicalTrials.gov, NCT01693601).
The only therapeutic approach for patients with advanced PMF, MPN-AP and MPN-AML that can eliminate the disease and provide long-term survival is SCT. In general, patients need to be younger and fit to undergo SCT; however, the high percentage of circulating blasts in patients with MPN-AP and MPN-AML significantly reduces the success of SCT (so much so that patients with blasts > 10% are deferred for SCT).[38] From our limited experience, it seems that in selected patients, the use of decitabine may result in a decrease in peripheral and bone marrow blasts, allowing the patient to become a candidate for transplant. This approach alleviates the need for an intensive AML-type chemotherapy, providing a safer alternative, and requires further study in clinical studies. We note that the outcome of transplant in our small group of patients was poor, with 4 of 7 dying in the first 100 days after transplant, highlighting not only a need for therapies that can better control disease and provide patients with the option of transplant, but also that transplant procedures themselves require significant improvements.[38]
In conclusion, decitabine is a viable therapeutic option for patients with very advanced MF or those in accelerated and blastic phase. Prospective clinical studies investigating the efficacy of decitabine in combination with other possibly active agents are warranted.
Highlights.
Decitabine has shown clinical activity in MPN-AML, MPN-AP and high risk PMF.
The responses observed with decitabine were durable.
Decitabine has shown possible improvement in the survival outcome of these patients.
Acknowledgments
This research is supported in part by a Cancer Center Support Grant from the National Cancer Institute (CA016672) to MD Anderson Cancer Center.
Footnotes
Talha Badar: Performed research, analyzed data and wrote the paper.
Hagop M. Kantarjian, Jorge E. Cortes, Farhad Ravandi, Elias Jabbour, Gautam Borthakur, Naveen Pemmaraju and Naval Daver: Contributed by providing patients data and helped in drafting the article.
Sherry R. Pierce: Contributed in data analysis.
Kate J. Newberry: Contributed in drafting the article.
Srdan Verstovsek: Supervised and provided the essential guidance in performing research, analyzing data and writing paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tefferi A. Primary myelofibrosis: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88(2):141–50. doi: 10.1002/ajh.23384. [DOI] [PubMed] [Google Scholar]
- 2.Mesa RA, et al. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–7. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 3.Gangat N, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–7. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27(27):4563–9. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefferi A, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108(4):1158–64. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, et al. Bortezomib therapy in myelofibrosis: a phase II clinical trial. Leukemia. 2008;22(8):1636–8. doi: 10.1038/leu.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesa RA, et al. A phase II trial of tipifarnib in myelofibrosis: primary, post-polycythemia vera and post-essential thrombocythemia. Leukemia. 2007;21(9):1964–70. doi: 10.1038/sj.leu.2404816. [DOI] [PubMed] [Google Scholar]
- 8.Abgrall JF, et al. Thalidomide versus placebo in myeloid metaplasia with myelofibrosis: a prospective, randomized, double-blind, multicenter study. Haematologica. 2006;91(8):1027–32. [PubMed] [Google Scholar]
- 9.Harrison C, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 10.Verstovsek S, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verstovsek S, et al. Three-year efficacy, overall survival, and safety of ruxolitinib therapy in patients with myelofibrosis from the COMFORT-I study. Haematologica. 2015 [Google Scholar]
- 12.Cervantes F, Mesa R, Barosi G. New and old treatment modalities in primary myelofibrosis. Cancer J. 2007;13(6):377–83. doi: 10.1097/PPO.0b013e31815a7c0a. [DOI] [PubMed] [Google Scholar]
- 13.Passamonti F, et al. Polycythemia vera in young patients: a study on the long-term risk of thrombosis, myelofibrosis and leukemia. Haematologica. 2003;88(1):13–8. [PubMed] [Google Scholar]
- 14.Tam CS, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood. 2008;112(5):1628–37. doi: 10.1182/blood-2008-02-138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti F, et al. Leukemic transformation of polycythemia vera: a single center study of 23 patients. Cancer. 2005;104(5):1032–6. doi: 10.1002/cncr.21297. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi AM, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–9. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- 17.Wang JC, et al. Hypermethylation of the P15INK4b and P16INK4a in agnogenic myeloid metaplasia (AMM) and AMM in leukaemic transformation. Br J Haematol. 2002;116(3):582–6. doi: 10.1046/j.0007-1048.2001.03319.x. [DOI] [PubMed] [Google Scholar]
- 18.Martyre MC. Critical review of pathogenetic mechanisms in myelofibrosis with myeloid metaplasia. Curr Hematol Rep. 2003;2(3):257–63. [PubMed] [Google Scholar]
- 19.Barosi G, et al. Diagnostic and clinical relevance of the number of circulating CD34(+) cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98(12):3249–55. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, et al. Correction of the abnormal trafficking of primary myelofibrosis CD34+ cells by treatment with chromatin-modifying agents. Cancer Res. 2009;69(19):7612–8. doi: 10.1158/0008-5472.CAN-09-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, et al. Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res. 2007;67(13):6417–24. doi: 10.1158/0008-5472.CAN-07-0572. [DOI] [PubMed] [Google Scholar]
- 22.Nischal S, et al. Methylome profiling reveals distinct alterations in phenotypic and mutational subgroups of myeloproliferative neoplasms. Cancer Res. 2013;73(3):1076–85. doi: 10.1158/0008-5472.CAN-12-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plimack ER, Kantarjian HM, Issa JP. Decitabine and its role in the treatment of hematopoietic malignancies. Leuk Lymphoma. 2007;48(8):1472–81. doi: 10.1080/10428190701471981. [DOI] [PubMed] [Google Scholar]
- 24.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15(12):3938–46. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian H, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 26.Silverman LR, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 27.Mascarenhas J, et al. Therapeutic options for patients with myelofibrosis in blast phase. Leuk Res. 2010;34(9):1246–9. doi: 10.1016/j.leukres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Thepot S, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM) Blood. 2010;116(19):3735–42. doi: 10.1182/blood-2010-03-274811. [DOI] [PubMed] [Google Scholar]
- 29.Quintas-Cardama A, et al. A phase II study of 5-azacitidine for patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia. 2008;22(5):965–70. doi: 10.1038/leu.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardiman JW, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 31.Mascarenhas J, et al. Proposed criteria for response assessment in patients treated in clinical trials for myeloproliferative neoplasms in blast phase (MPN-BP): formal recommendations from the post-myeloproliferative neoplasm acute myeloid leukemia consortium. Leuk Res. 2012;36(12):1500–4. doi: 10.1016/j.leukres.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tefferi A, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–8. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheson BD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 34.Verstovsek S, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–71. doi: 10.3324/haematol.2013.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eghtedar A, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119(20):4614–8. doi: 10.1182/blood-2011-12-400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabarroki A, et al. Ruxolitinib in combination with DNA methyltransferase inhibitors: clinical responses in patients with symptomatic myelofibrosis with cytopenias and elevated blast(s) counts. Leuk Lymphoma. 2014:1–3. doi: 10.3109/10428194.2014.916805. [DOI] [PubMed] [Google Scholar]
- 37.Mascarenhas J, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF) Br J Haematol. 2013;161(1):68–75. doi: 10.1111/bjh.12220. [DOI] [PubMed] [Google Scholar]
- 38.Ballen KK, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16(3):358–67. doi: 10.1016/j.bbmt.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]