Figure 1. IL-4 and IL-13 receptor structure.
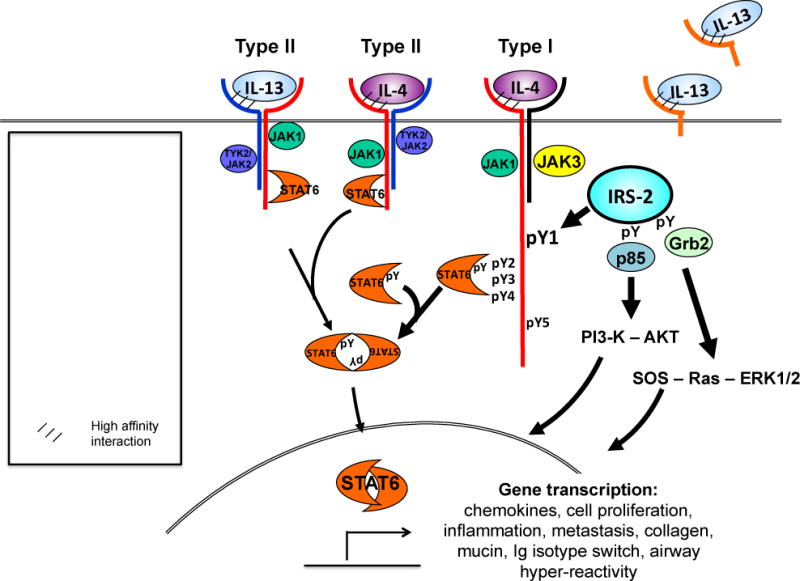
IL-4 signals through two possible receptor complexes composed of a heterodimer of the IL-4Rα (140 kDa) and γc chain (60 kDa); type I receptor or the IL-4Rα and IL-13Rα1 (65–70 kDa) chain; type II receptor. IL-4 binds IL-4Rα with high affinity, triggering dimerization with the secondary signaling chain. IL-13 binds IL-13Rα1 which complexes with the IL-4Rα, forming the type II receptor. Signaling through the type I receptor leads to activation JAKs and downstream signaling adaptor molecules STAT6 and IRS-2, whereas signaling through the type II receptor predominantly activates STAT6. IL-4 signaling also activates PI3-K and AKT. IL-13 also binds cell-surface and soluble forms of the IL-13Rα2, this so-called inhibitory subunit binds IL-13 with greater affinity than IL-13Rα1 and acts as a “cytokine sink”.
